Trabecular Juvenile Ossifying Fibroma Management by Virtual Surgical Planning, Piezoelectric Surgery, and Simultaneous Patient Specific Implant (PSI) Reconstruction
February 28, 2020
https://doi.org/10.23999/j.dtomp.2020.2.6
J Diagn Treat Oral Maxillofac Pathol 2020;4(2):39−48.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE
Aldana H, Orozco M, Ordoñez L, Estrada CI, Mosquera C. Trabecular juvenile ossifying fibroma management by virtual surgical planning, piezoelectric surgery, and simultaneous patient specific implant (PSI) reconstruction. J Diagn Treat Oral Maxillofac Pathol 2020;4(2):39–48.
Contents: Summary | Introduction | Case | Discussion | Conclusions | Patient Consent | Conflict of Interests | Fundings | Acknowledments | References (20)
Summary
Trabecular juvenile ossifying fibroma is a rare fibro-osseous lesion affecting the craniofacial skeleton occurring commonly in children and young adults. Tumor clinical behavior is highly aggressive with invasion of adjacent anatomic structures. Because of its high recurrence rate complete excision is necessary, but this one could be facial mutilating. This case report presents a 23-year-old female patient with a trabecular juvenile ossifying fibroma of the right maxilla, expanding into the orbit and zygomatic bone. The report also shows the multidisciplinary surgical management of this lesion with successful preservation of optic nerve function and facial aesthetics.
Introduction
Ossifying fibroma is a benign fibro-osseous neoplasm characterized by progressive bone expansion, locally aggressive and recurrent behavior that can occur in the bones of the craniofacial complex.1 Microscopically it is characterized by the replacement of medullary bone by fibrous tissue with varying amounts of immature or cementoid bone.1 According to the World Health Organization (WHO), it is classified in three forms: Conventional ossifying fibroma, psamomatoid juvenile ossifying fibroma, and trabecular juvenile ossifying fibroma. The last one is an infrequent lesion characterized by its presentation in facial bones different from the maxillary bones, with rapid growth and highly deforming aspect.2 It occurs mostly in patients between the ages of 20-40 years, although it may present in children and adolescents as well as in older adults. Females are more commonly affected than males with a ratio of 5:1. The treatment consists of its radical excision due to the high rates of relapse (30-50%),2 this resection can be mutilating, especially because of the size, the location close to important structures and the difficult surgical access, which implies appearance of facial aesthetics and functional sequels in patients.3
The purpose of this case report is to show a multidisciplinary and minimally invasive surgical management in a young patient who was diagnosed with trabecular juvenile ossifying fibroma in the right zygomatic-orbito-maxillary region.
Case
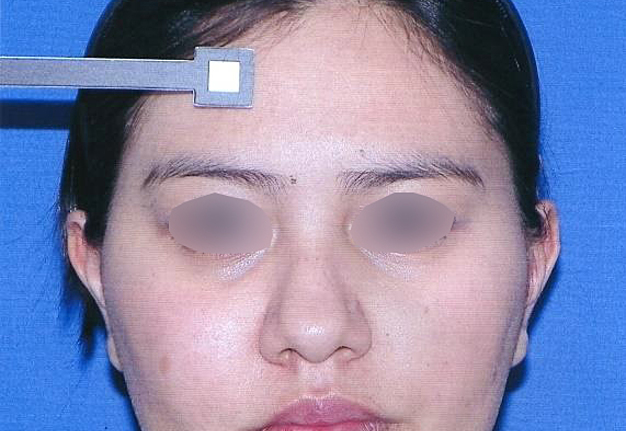
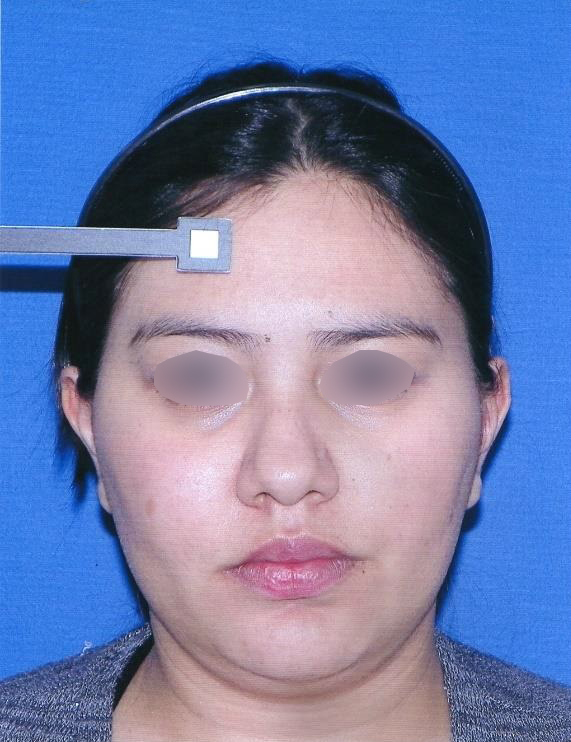
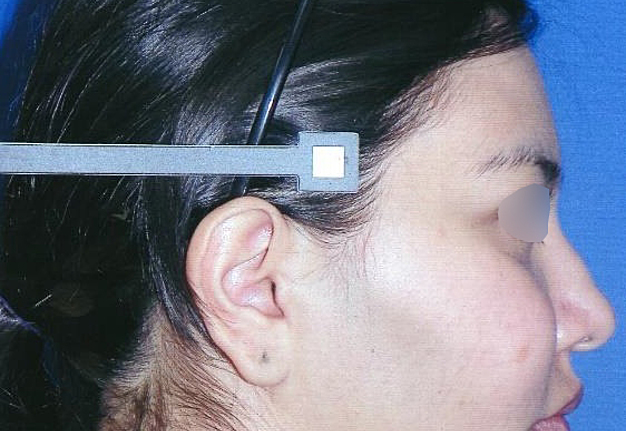
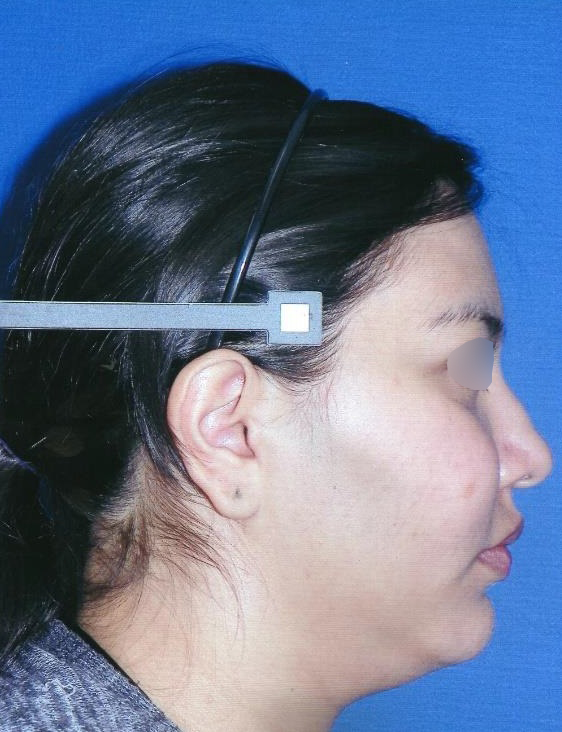
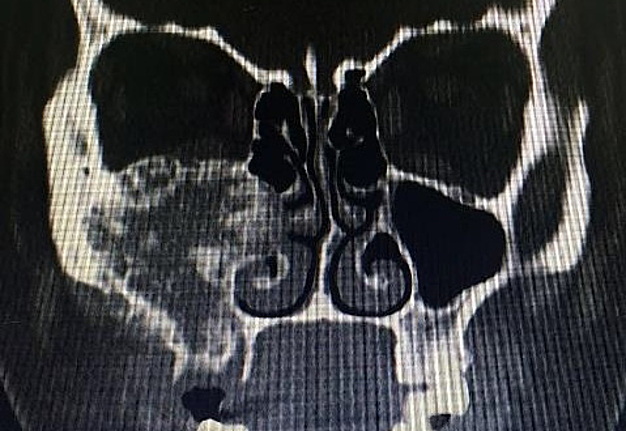
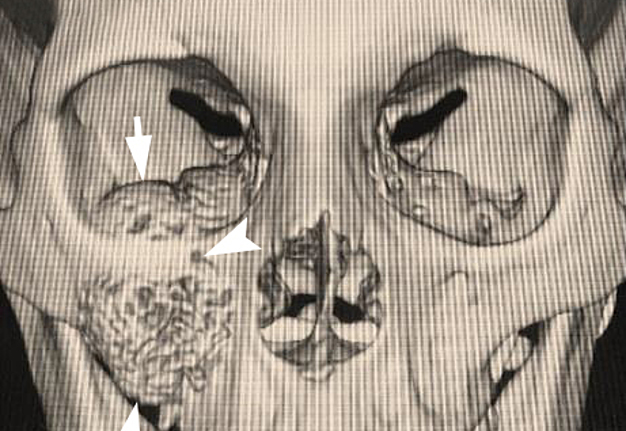
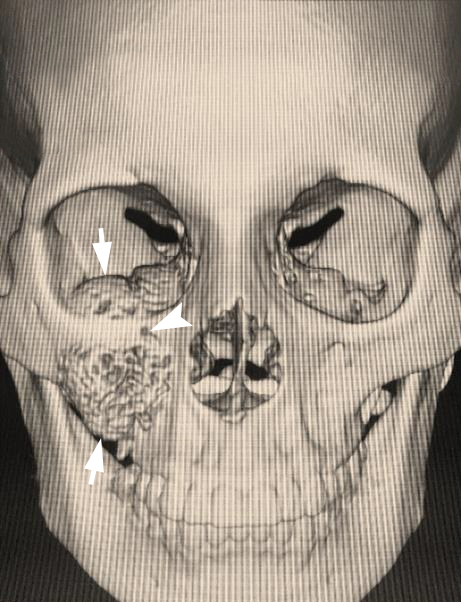
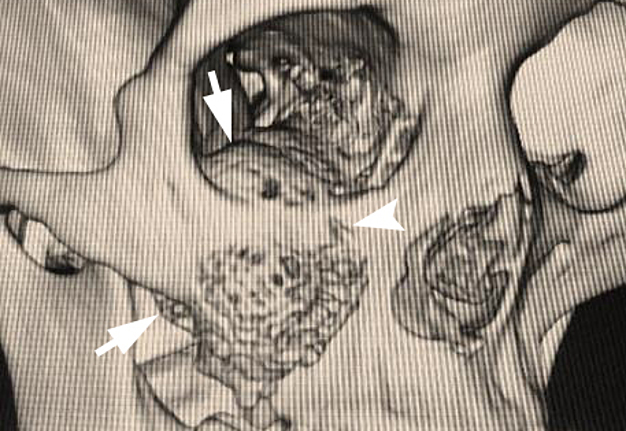
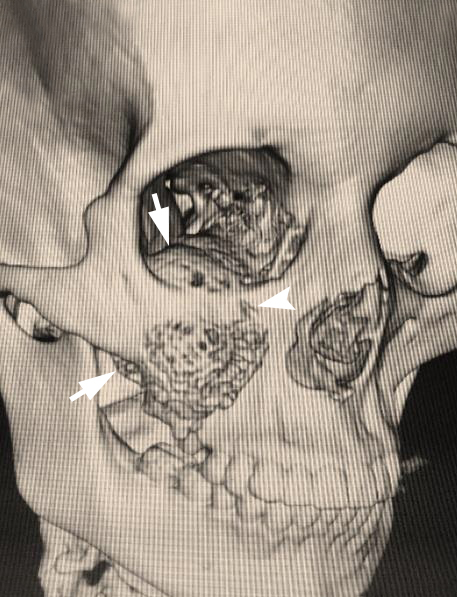
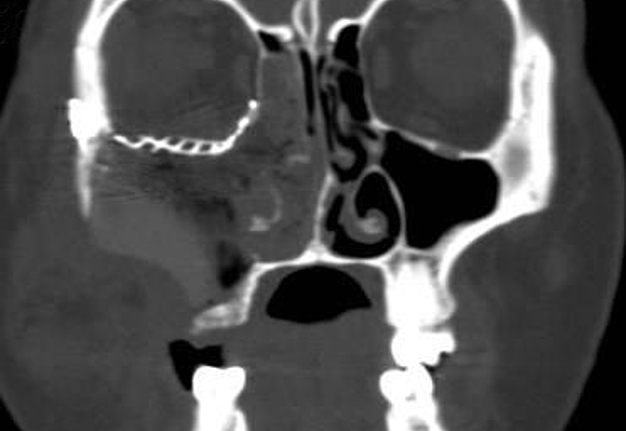
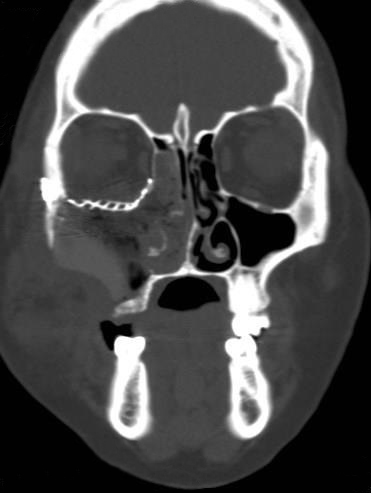
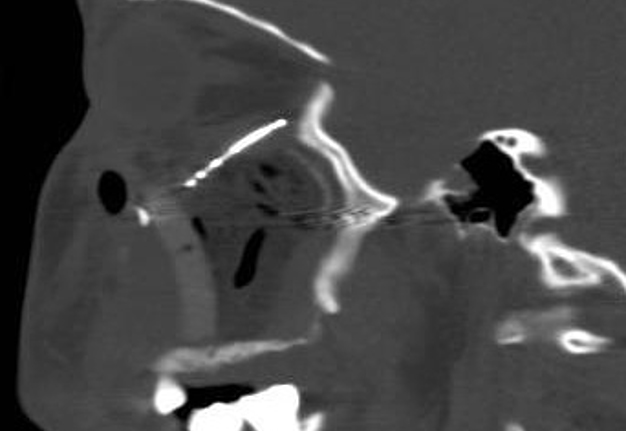
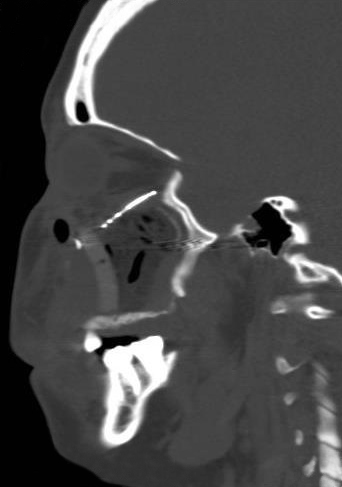
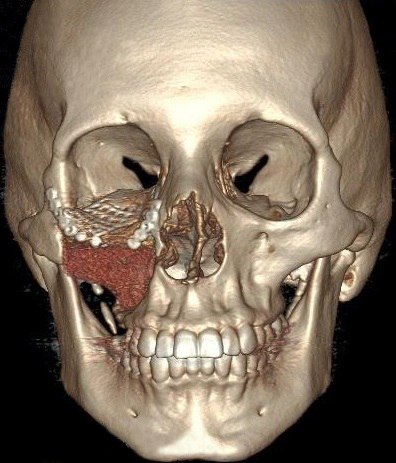
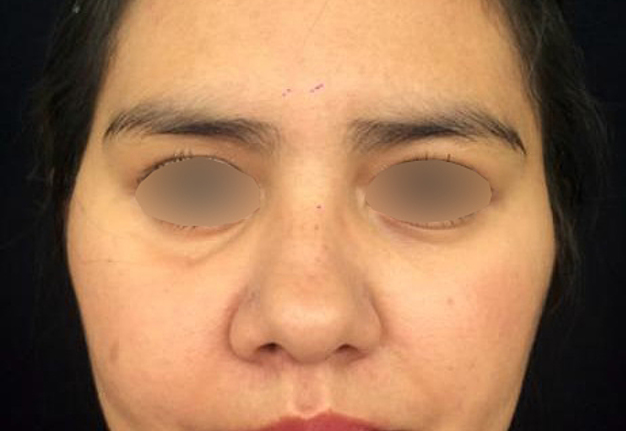
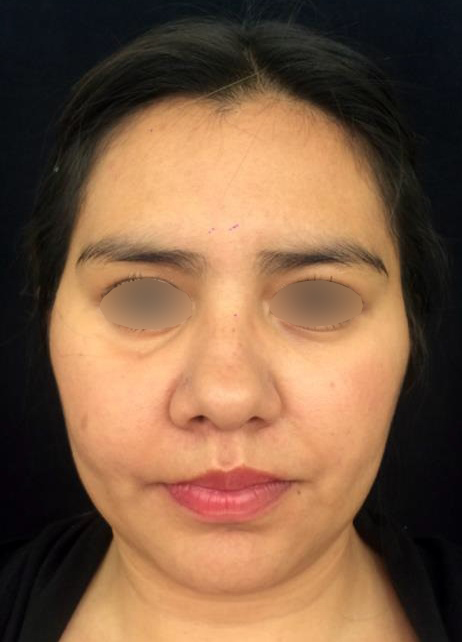
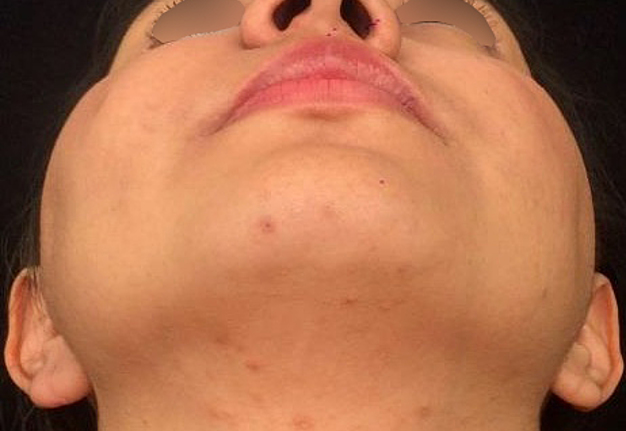
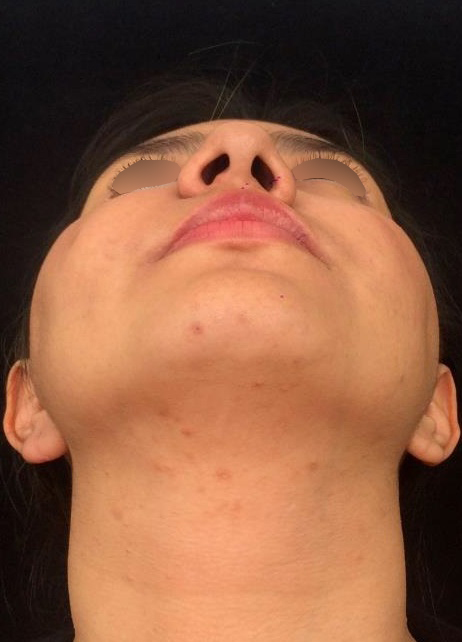
A 23-year-old female patient, with no history of illness, was presented with a painless, progressive swelling of the rigth malar region of indetermineted evolution (Fig 1). The clinical evaluation shows an indurated contour deformation that extends from right inferior orbital rim, right zygomatic bone, and anterior wall of right maxillary sinus to ipsilateral maxilla; 20/20 visual acuity and eye movements are preserved. Computed tomography (CT) imaging study revealed an intra-osseous lesion in right orbitozigomatic region with irregular borders and cortical expansion, which extends in orbit floor, with close proximity to the right orbital apex, lateral nasal wall, which extends in its lower limit to the pterygopalatine fossa (synonym: pterygomaxillary fossa)4 and ipsilateral posterosuperior alveolar ridge; in the front lesion was invading anterior wall of maxillary sinus to the alveolar ridge, approximately 50 × 40 × 40 mm in length (Fig 2). Firstable incisional biopsy of the lesion is performed and histopathological diagnosis of “trabecular juvenile ossifying fibroma” is obtained.
After a critical analysis of the case in a surgical meeting of the department of oral and maxillofacial surgery in conjunction with the otorhinolaryngology department in Colombia University Clinic we decided to perform a radical surgical excision of the tumor using piezoelectric surgery in order to avoid injury to nearby vital structures such as the optic nerve and the contents of the pterygopalatine fossa. Also the immediate placement of patient specific implant (PSI) is planned for simultaneous implantation in same surgery time to improve the effect of operation. By last orbit floor reconstruction is planned in conjunction with endoscopic guide.
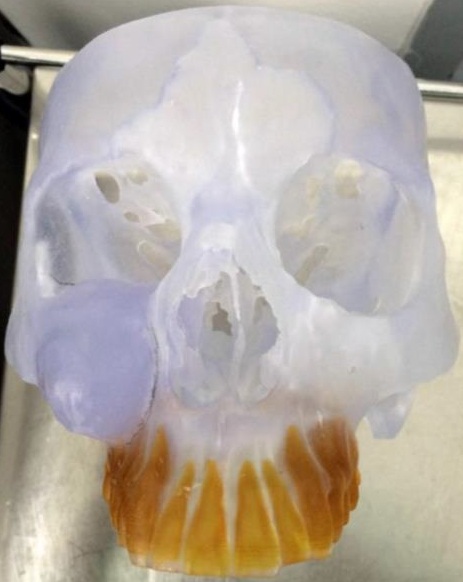
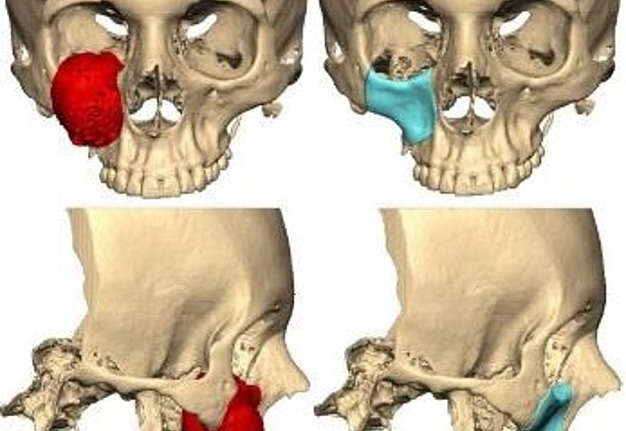
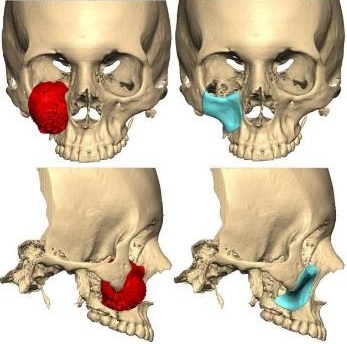
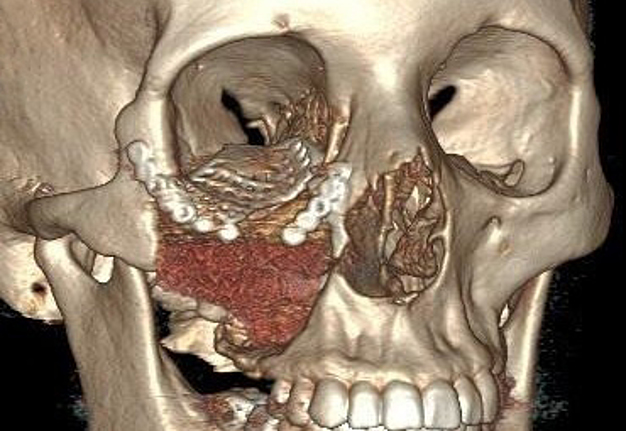
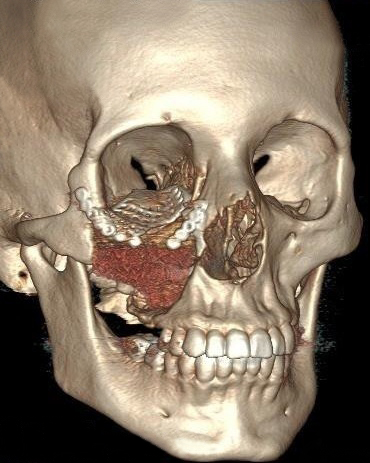
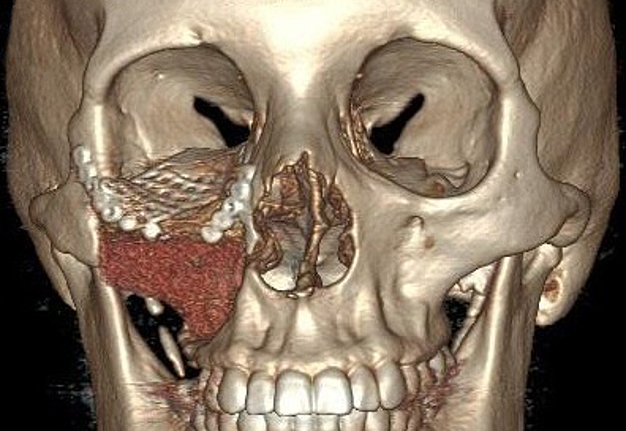
The patient underwent preoperative computed tomography scan (CT) of the cranio-facial complex before surgery to produce a replica with stereolithographic model using computer-aided design/computer-aided manufacturing technology (CAD/CAM) (Fig 3A). Preoperative surgical planning was done based on this model, to determine the extent of excision and surgical approaches. We perform preoperative virtual surgical planning; the CT images were imported to Synthes ProPlan CMF (Materalise NV, Leuven, Belgium). Then tumor resection was simulated on the computer by engineers and the surgical team to determine the extent of the osteotomy and the location of osteotomy line (Fig 3B). The three-dimensional mirror image of the contralateral unaffected side was used for reconstruction of the defect. Based
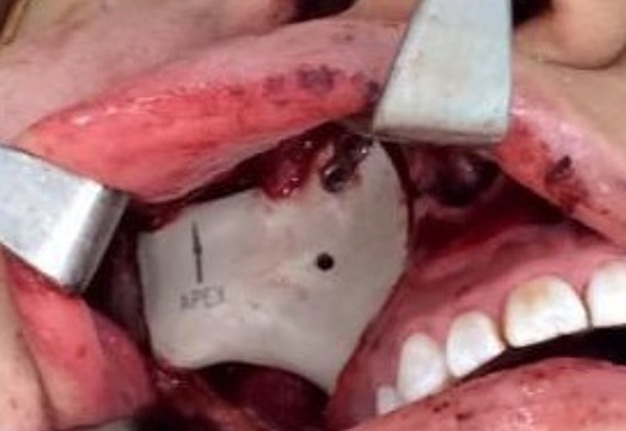
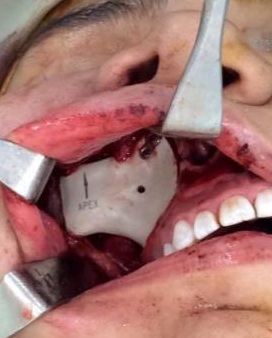
on the mirror image, a PSI was manufactured in polyether-ether-ketone (PEEK) biomaterial (AO CMF, Synthes, Solothurn, Switzerland) (Fig 3C) which was used to rehabilitate the contour of the zygoma, anterior wall of the maxilla and inferior orbital rim. For reconstruction of the right orbital floor we decided to use a preformed orbital plate, MatrixOrbital, MatrixMIDFACE (DePuy Synthes, Solothurn, Switzerland) (Fig 3D). A day before the surgery patient went to selective embolization of right maxillary artery to avoid massive bleeding in the intervention.
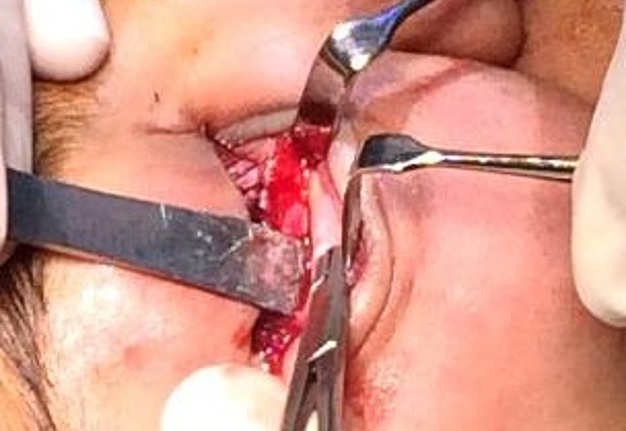
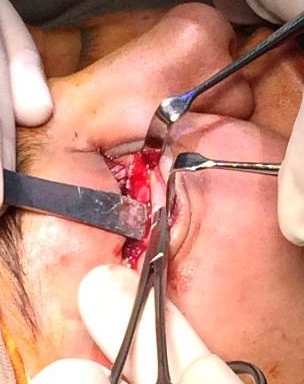
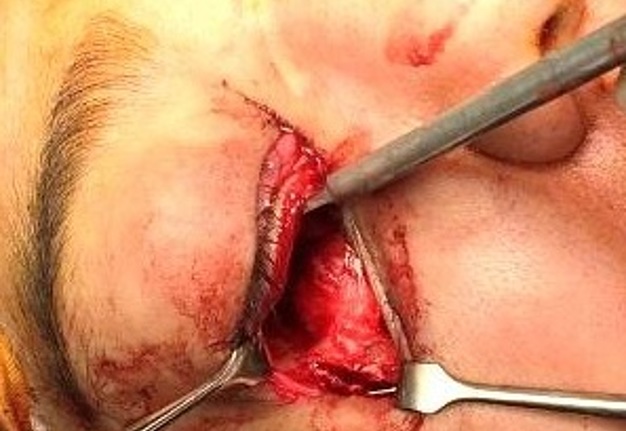
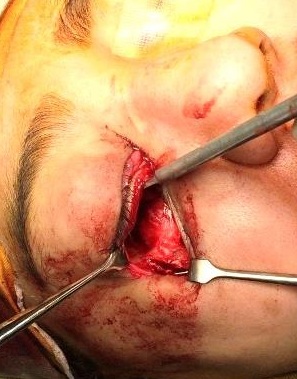
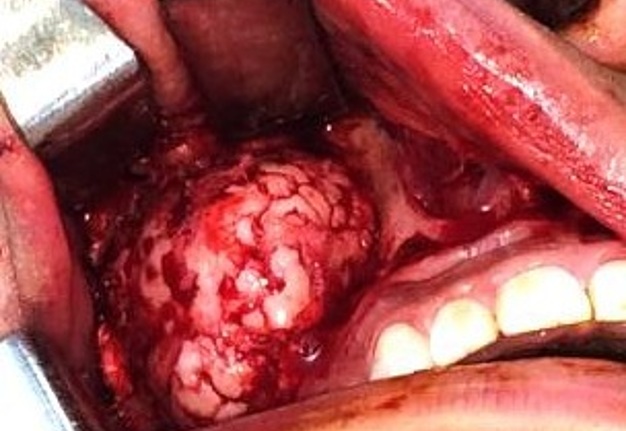
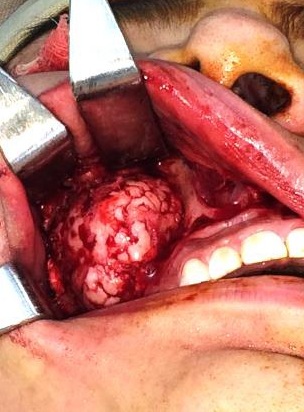
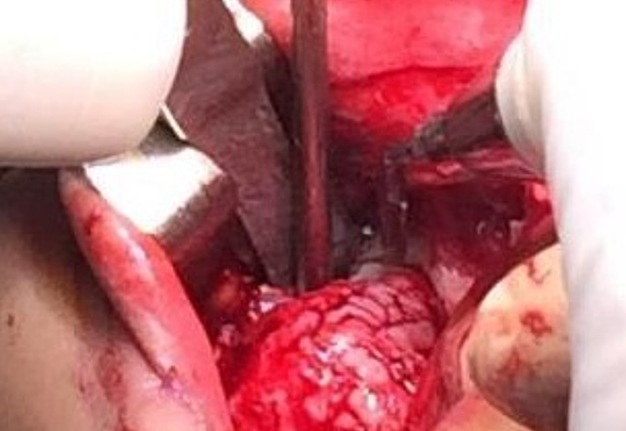
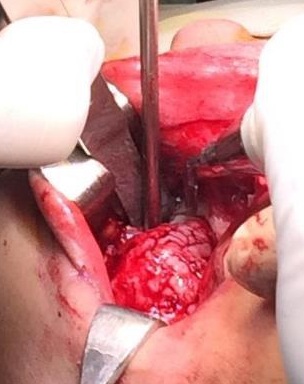
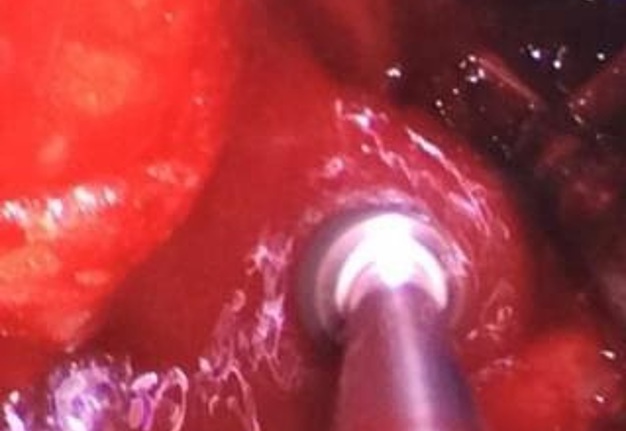
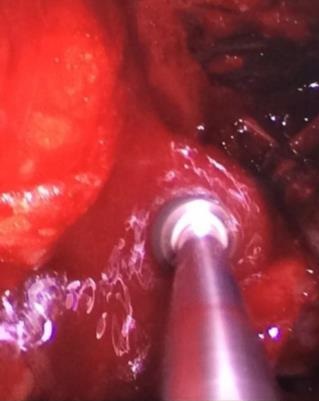
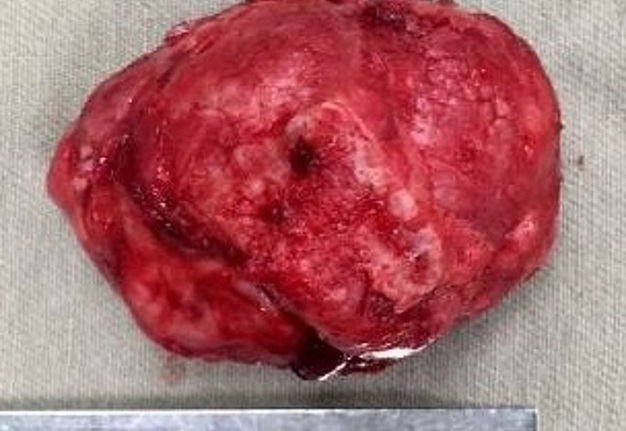
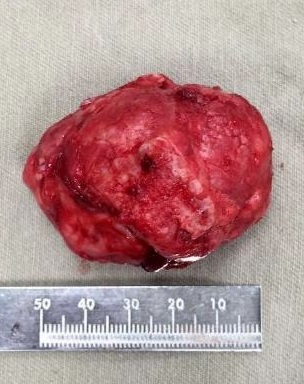
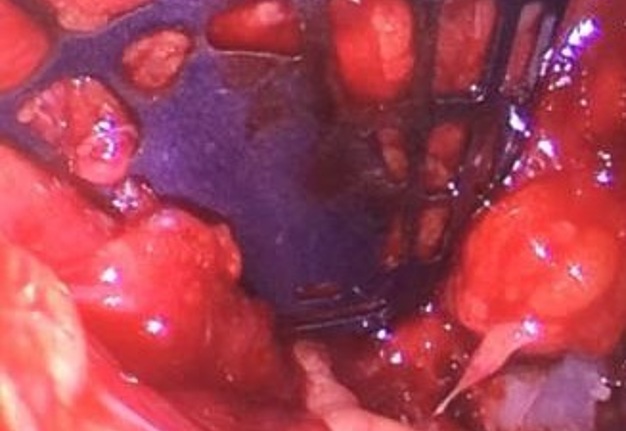
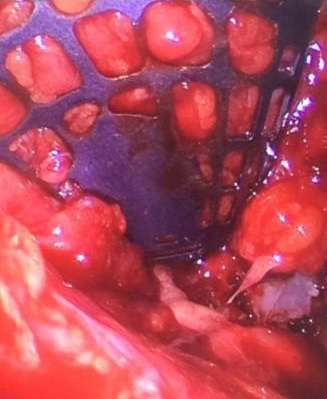
The patient was prepared and taken for surgery under general anesthesia (Fig 4), the tumor was totally resected using a combined palpebral (subciliary) and intraoral approach to the right upper buccal sulcus (Fig 4A). A temporary tarsorraphy was performed for the rigth eyelid. The orbital floor was dissected subperiosteally and the globe retracted superiorly visualizing the entire floor (Fig 4B). Conecting subciliary incision with intraoral incison the lesion was totaly exposed (Fig 4C) and the osteotomy cuts were marked on the bone, according to the virtual plan aided with stereolithographic model. The osteotomy was realized with Surgybone (W. Lorenz, Bogotá, D.C., Colombia) (Fig 4D) piezoelectric surgery unit permitting a selective cut of mineralized tissue while sparing soft tissue. After visible tumor was removed (Fig 4F), endoscopic examination of temporay cavity was perform by the otorhinolaryngology specialist; he was in charge of the removal of the tumor remains present in the nasal lateral wall and in the osseous remnant of the orbital floor near apex with Midas high-speed surgical drill (MediRex Inc., CA, USA) (Fig 4E). For the reconstruction of the large bony defect PEEK PSI (Fig 4G) was fixed in zygomatic and maxillary remaining bone using MatrixMIDFACE 0.4-mm plates and 5-mm screws (DePuy Synthes, Solothurn, Switzerland). For the right orbital reconstruction was put a preformed orbital plate MatrixOrbital (DePuy Synthes, Solothurn, Switzerland) fixed with 5-mm screws to the PEEK PSI.
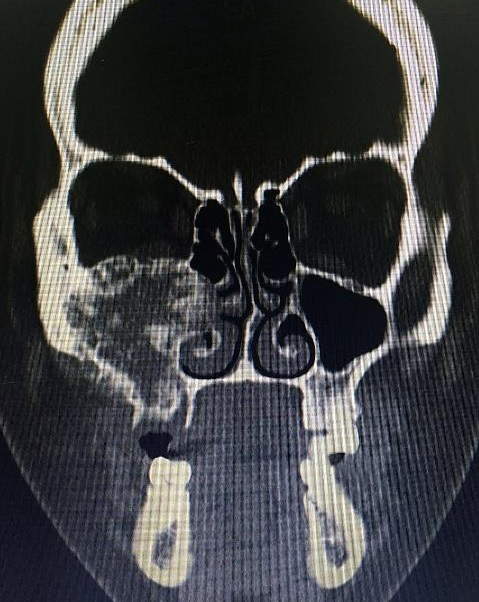
In the part of the osseous remnant of the orbital apex, we placed the mesh with the help of an endoscopic guide to make sure that the optic nerve was not harmed at any time and to guarantee the osseous support of the same (Fig 4H). A follow-up CT was obtained a day after the intervention (Fig 5), evidencing adequate restoration of the right zygomatic-orbitomaxillary region and facial contour. One year after surgery patient is stable with ophthalmologic examination within normal limit (Fig 6).
Discussion
Trabecular juvenile ossifying fibroma is a wellcircumscribed tumor with slow-growing and well demarcated from adjacent bone. The lesion which is consisting of proliferating fibroblasts and osseous products that include bone and cementum-like material. Surgical excision is the principal treatment of ossifying fibroma, small and well-demarcated lesions can be treated by conservative surgery (curettage and enucleation) along with long-term clinical and radiographic follow-up. Some lesions may grow to massive size, causing considerable esthetic and functional deformity,5, 6, 7 this kind of lesion requires radical surgery in form of resection and reconstruction, and periodic radiographic monitoring as well. Mohanty et al7 in a 10 years retrospective study, found 25 cases with clinical, radiological and histopathological features of ossifying fibroma of jaw bones. It also showed that the treatment rendered in the form of enucleation, curettage or resection of the lesion depending on its stage and extent were adequate, as no recurrence has been reported.7 Titinchi et al8 in another paper show data from the reported literature an average recurrence rate of 10.1% with an average follow-up period of 25.3 months.
Multi-disciplinary team facilitates safe resection of these difficult tumors like was reported for Hachach-Haram and Hartstein.3, 9 It is very important to thoroughly evaluate the case to perform an adequate
prior surgical planning. With the advent of new technologies, the three dimensional virtual planning using a combination of a stereolithographic model and navigation system, can greatly aid in the making of relevant decisions in the design of osteotomies and in the prevention of injuries to vital structures close to the tumor.10, 11
Ultrasonic waves are used in oral and maxillofacial surgery for various diagnostic and therapeutic procedures. They are applied in diagnostics, endodontics, the removal of calculus from the teeth and, most recently, osteotomies with piezoelectric devices.12,13 This case show one of the indications for the use of piezoelectric devices in resection tumor surgery and the beneficial effects of this technique in bone cutting close to vital structures for avoing damage. In 2000, Vercellotti presented an ultrasound osteotomy a novel technique for osteotomy without damage to adjacent soft tissue.14 Piezoelectric substances have the capacity to be deformed when placed into an electric field. If the polarity of the field changes periodically, these materials start vibrating. Ultrasonic vibrations can then be transmitted to diverse solid, liquid or gaseous materials. This property is used in ultrasonic scalers with a functional frequency of around 20 kHz. Addition of a 50 kHz pulse every 10 ns to this basal frequency increases the power of the receiver device, allowing it to cut bones accurately without damaging soft tissues for example nerves and blood vessels.12, 13 In this specific case the ossifying fibroma tumor was very close to the right optic nerve and to the ipsilateral pterygopalatine fossa. Therefore, the performance of osteotomies with piezoelectric surgery decreased the risk of intraoperative complications and postoperative functional sequelae. Besides another advantage in intraoperative use of piezoelectric devices is the maintenance of blood-free operative area secondary to the physical phenomenon of a cavitation effect from the continuous irrigation solution. This permits great intraoperative visible control with the consequent increase in safety, especially in anatomically difficult areas.12, 13
Another important item is the extension of the defect left by tumor resection and the different techniques chosen for reconstruction. Numerous autogenous and alloplastic materials have been used in maxillofacial reconstruction considering the advantages and disadvantages of each material in a given clinical situation. Autogenous bone has been considered the gold standard for osseous reconstruction and is still widely used. Grafts become vascularized and osseointegrate with surrounding bone, minimizing the risk of infection and rejection. Nevertheless, harvest requires added operative time and donor-site morbidity. Autogenous bone has unpredictable resorption and can be difficult to mold to meet the requirements of the craniofacial skeleton. In addition, the supply of autogenous graft is limited.15 Many alternatives to autogenous bone graft have become available and share two advantages; the supply is limitless, and a donor site is not required. Some materials can be molded or custom manufactured to fit the bone deformity.15, 16
There are many kind of alloplastic materials and PSI is a personalized approach to reconstructive surgery. This is particularly useful in maxillofacial surgery in which restoring the complex 3-dimensional
contour. Recent advances in computer aided design/computer-aided manufacturing (CAD/CAM) have created innovative options for fabricating PSI, with improved precision, better adaptation that improves contour outcomes.16 Maxillofacial PSIs are commonly manufactured from metals and polymers; in this particular case the PSI was manufactured in polyether-ether-ketone (PEEK) biomaterial. PEEK, is a member of the polyaryletherketone (PAEK) polymer family that has been used for orthopedic and spinal implants. This material is a semicrystalline polymer which is thermally processable, with excellent biocompatibility, has high chemical resistance and fatigue, good rigidity and hardness, can be sterilized several times, without any significant degradation of its properties, tolerating temperatures up to 200°C.17 Additionally presents compatibility with many reinforcing agents, such as glass and fibers, and increased strength in function of the weight, compared with that of other metals.17 Among its advantages are
evidenced a rigidity and resistance similar to that of the cortical bone and radiographic translucency. One of the main disadvantages of PEEK implants, is that of postoperative complications, namely infection; despite this PEEK has demonstrated good outcomes both esthetically and functionally, with a complication rate similar to that of other alloplastic materials.18, 19 This material is widely recommended
in selected cases with large or complex defects in the maxillofacial area, as evidenced in this case.20
Conclusions
Although the use of computer-designed PEEK PSI in the rehabilitation of the maxillofacial area remains restricted for the moment in some areas of Latin America, the knowledge and implementation of new and increasingly conservative surgical techniques allow us to solve complex cases in ever simpler ways. The successful outcome in this case was due to a combination of a multidisciplinary team approach,
precise pre-operative planning, and the use of a novel surgical technique. The results can offer improvement in quality of life in persons avoiding suffer from significant facial deformity.
Patient Consent
The patient provided written consent of the use of her images.
Conflict of Interests
The authors declare no conflict of interest.
Fundings
No funding was received for this study.
Acknowledments
None.
References (20)
- Colmenero-Ruiz C, Cano-Sánchez J, López-Arcas JM, Martínez-Iturriaga MT, Campo-Trapero J, Castelló-Fortet JR. Multistage reconstruction in facial juvenile psammomatoid ossifying fibroma: clinical therapeutic conference. J Oral Maxillofac Surg 2011;69(7):2055–63.
- Zegalie N, Speight PM, Martin L. Ossifying fibromas of the jaws and craniofacial bones. Diagn Histopathol 2015;21(9):351–8.
- Hachach-Haram N, Benyon S, Maling S, Joshi N, Grant WE, Kirkpatrick WN. Surgical management of two complex cases of large juvenile orbital ossifying fibroma. J Plast Reconstr Aesthet Surg 2011;64(12):1661–4.
- Montgomery WW, Katz R, Gamble JF. Anatomy and surgery of the pterygomaxillary fossa. Ann Otol Rhinol Laryngol 1970;79(3):606–18.
- Lv M, Li J, Shen Y, Wang L, Sun J. The “drawer-like” resection and reconstruction with titanium mesh: a novel surgical technique for treatment of giant ossifying fibroma in the maxilla. J Oral Maxillofac Surg 2017;75(8):1752–61.
- Triantafillidou K, Venetis G, Karakinaris G, Iordanidis F. Ossifying fibroma of the jaws: a clinical study of 14 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114(2):193–9.
- Mohanty S, Gupta S, Kumar P, Sriram K, Gulati U. Retrospective analysis of ossifying fibroma of jaw bones over a period of 10 years with literature review. J Maxillofac Oral Surg 2014;13(4):560–7.
- Titinchi F, Morkel J. Ossifying fibroma: analysis of treatment methods and recurrence patterns. J Oral Maxillofac Surg 2016;74(12):2409–19.
- Hartstein MR, Grove AS, Woog JJ, Shore JW, Joseph MP. The multidisciplinary management of psammomatoid ossifying fibroma of the orbit. Ophthalmology 1998;105(4):591–5.
- Farrell B, Franco PB, Tucker MR. Virtual surgical planning in orthognathic surgery. Oral Maxillofac Surg Clin N Am 2014;26(4):459–73.
- Arai Y, Chiba Y, Umeda S, Ohara Y, Iwai T, Komatsu M, Yabuki K, Sano D, Oridate N. Reduction surgery using a combination of a stereolithographic model and navigation system for ossifying fibroma with secondary central giant cell granuloma. Auris Nasus Larynx 2016;43(2):207–11.
- Beziat JL, Bera JC, Lavandier B, Gleizal A. Ultrasonic osteotomy as a new technique in craniomaxillofacial surgery. Int J Oral Maxillofac Surg 2007;36(6):493–500.
- Wagner ME, Rana M, Traenkenschuh W, Kokemueller H, Eckardt AM, Gellrich NC. Piezoelectric-assisted removal of a benign fibrous histiocytoma of the mandible: an innovative technique for prevention of dentoalveolar nerve injury. Head Face Med 2011;7:20.
- Vercellotti T. Piezoelectric surgery in implantology: a case report – a new piezoelectric ridge expansion technique. Int J Periodontics Restorative Dent 2000;20(4):358–65.
- Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg 2012;23(1):323–7.
- Owusu JA, Boahene K. Update of patient-specific maxillofacial implant. Curr Opin Otolaryngol Head Neck Surg 2015;23(4):261–4.
- Kurtz S. An overview of PEEK biomaterials. In: Kurtz SM, editor. PEEK biomaterials handbook, 1st ed. William Andrew: Elsevier. 2012:1–8.
- Lovald S, Kurtz S. Applications of polyetheretherketone in trauma, arthroscopy, and cranial defect repair. In: Kurtz SM, editor. PEEK biomaterials handbook, 1st ed. William Andrew: Elsevier. 2012:243–60.
- Alonso-Rodriguez E, Cebrián JL, Nieto MJ, Del Castillo JL, Hernández-Godoy J, Burgueño M. Polyetheretherketone custom-made implants for craniofacial defects: report of 14 cases and review of the literature. J Craniomaxillofac Surg 2015;43(7):1232–8.
- Scolozzi P. Maxillofacial reconstruction using polyetheretherketone patient-specific implants by ‘‘mirroring’’ computational planning. Aesth Plast Surg 2012;36(3):660–5.