Influence of the Neural Crest-derived Stem Cells to Optic Nerve Regeneration After Its Experimental Injury
August 02, 2021
https://doi.org/10.23999/j.dtomp.2021.8.2
J Diagn Treat Oral Maxillofac Pathol 2021;88–96.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE
Chepurnyi YV, Kopchak AV, Korsak A, Likhodievskyi V, Chaikovskyi Y, Zlatska A, Gubar O, Gordiienko I, Rodnichenko AE. Influence of the neural crest derived stem cells to optic nerve regeneration after its experimental injury. J Diagn Treat Oral Maxillofac Pathol 2021;5(8):88–96.
ABSTRACT
Purpose: Orbital trauma is a challenging problem due to such severe sequel as diplopia, decrease of vision or eye motility disorder. However, the conditions of orbital soft tissue content still become underestimated. The aim of this study was to investigate structural changes in the rat optic nerve after experimental injury followed by treatment with stem cells.
Materials and Methods: An experimental model of injury to the orbital soft tissue content in the rat was developed. Forty Wistar rats maintained under daylight were divided into two equal experimental groups. Unlike the rats of Group I, in rats of Group II, the site of injury to the orbital soft tissue mass received postnatal multipotent stem cells, epidermal neural crest stem cells (NCSCs) derived from the bulge of hair follicles.
Results: Comparing the number of glial cells per certain area of the slice (NC) between group І and site without injury (control) after 3 week of observation, it was higher in group I more than 258.8% (p < 0.0001) and on 272.4% in group II (p < 0.0001). After 6 weeks NC in group I was higher than at previous terms: more then 128.9% (р < 0.0001). At the same, NC in group II was higher comparing with previous terms only on 17.1% (р = 0.0212). Between the animals of group I at terms of 12 and 24 weeks NC high and wasn’t significantly differ between this terms of observation (ANOVA p = 0.4379). In contrast, NC in group II stopped rising between 6 and 12 weeks demonstrating statistical equality (p = 0.4563).
Conclusions: It can be assumed that the application of mesenchymal stem cells, derivates of the neural crest, after the experimental orbital trauma, stimulates a recovery of the optic nerve. Further studies should be performed to more deeply discover the neural crest derived stem cell populations, ivoleved into recovery of damaged optic nerves.
INTRODUCTION
Orbital trauma still become a challenging problem due to such severe sequel as diplopia, decrease of vision or eye motility disorder. All mentioned sequelae dramatically decrease the quality of life of the patients, influencing their social activity and lead in some cases to partial or total professional disability.1–3 In most cases, orbital trauma results from the direct or indirect injuries of orbital bony structures, as well as orbital soft tissue content. Orbital wall fractures lead to appearance of the bony defects requiring reconstruction. Besides, soft tissue structures may be injured by the little bony fragments or due to compression and hypoxia of increasing swelling or hematoma.4-6
Usually, much attention is paid to precise reconstruction of the orbital walls, when the conditions of the soft tissue content remains underestimated. Such nervous structures as cranial nerves and optic nerve can be damaged with loose of their inherent function and following functional disability. Injuries to the organs of the nervous system have been especially difficult to treat due to their potential for regeneration.7,6 Although numerous relevant studies have been conducted, and the pathogenesis of optic nerve damage has been thoroughly investigated, effective methods to promote regeneration of damaged optic nerve are still to be developed.2, 8–11 Due to this, therapeutic influence to the regeneration of the nervous structures of the orbital soft tissue content could be recognized as appropriate component of complex orbital trauma management in addition to surgical reconstruction. However, treatment strategies capable of effecting major phases of the regenerative process should be developed. The use of stem cells for this purpose has been found especially promising.12
Among the variety of the different types of stem cells, used in regenerative medicine, the adult neural crest-derived stem cells (aNCSCs) could be the most promising ones, concerning regeneration of orbital soft-tissue content. According to Blentic et al13 a significant part of the maxilla-facial skeleton, including bones of the orbit except minor wing of the sphenoid, originates from ectomesenchyme – derivate of neural crest. After specification and migration to target tissues and organs the neural crest cells originate different types of tissues, including the bone, cartilage and connective tissue in the head and neck region, neurons and glia of the peripheral nervous system, melanocytes, endothelial cells etc. The main advantages of aNCSCs, mentioned in the literature, are capability to most wide-ranging multilineage differentiation and the plasticity of the HOX code, which allows modifying their original one after transplantation into the damaged tissue site, acquiring the characteristic of recipient tissues’ HOX code. Due to above mentioned, therapeutic application of the aNCSCs allows to suspect the restoration of a bone,14,15 as well as damaged nerves and muscles, which makes them attractive candidates for application in regenerative medicine,16,15 especially in the field of orbital trauma managements.
The aim of this study was to investigate structural changes in the rat optic nerve after experimental injury followed by treatment with stem cells.
MATERIALS AND METHODS
This study was performed in accordance with the ethical standards of the institutional and national research committees, the laws of Ukraine and the 1964 Helsinki declaration and its later amendments. The study protocol was approved by the Bogomolets National Medical University Bioethics Committee (Protocol No 126).
An experimental model of injury to the orbital soft tissue content in the rat was developed. Forty Wistar rats (weight, 180-220 g) maintained under daylight were divided into two equal experimental groups. With the animal under thiopental sodium anesthesia (50 mg/kg, intraperitoneally), the orbital soft tissue content was injured by separation of the fat tissue and oculomotor muscles with following clamping with forceps of the middle third of the intraorbital portion of the optic nerve for 30 seconds.17 Unlike the rats of Group I, in rats of Group II, the site of injury to the orbital soft tissue mass received postnatal multipotent stem cells, epidermal neural crest stem cells (eNCSCs) derived from the bulge of hair follicles.18 The surgical wound was sutured in layers.
At weeks 3, 6, 12 and 24 after experimental injury, the optical nerve (intraocular portion, intraorbital portion, intracanalicular portion and intracranial portion going up to the optic chiasma) was taken for study and compared with that of the normal (control) contralateral orbit. Animals were euthanized with an overdose of thiopental sodium prior to taking the material for study. The material was fixed in 10% neutral phosphate buffered formalin. Hematoxylin and eosin staining, silver nitrate impregnation, and Spielmeyer staining were used to investigate the structure of the optic nerve. Optic nerves were washed in phosphate buffer solution. Sections of the optic nerve were cut on a freezing microtome and impregnated by the silver nitrate method (“rapid method for impregnation of the components of the peripheral nervous system with silver nitrate”).19 In addition, the material was stained for the demonstration of myelin sheaths by the Spielmeyer method. After conventional histological processing, the optic nerve was embedded in paraffin, and longitudinal and transverse sections were cut serially and stained with hematoxylin and eosin. Photographs were taken with a digital camera (C-4040 Zoom; Olympus, Tokyo, Japan) attached to a light microscope (Olympus model BX51). ImageJ 1.51a (National Institutes of Health [NIH], Bethesda, Maryland, United States; http://rsbweb.nih.gov/ij) was used for the analysis of digitized images.
For morphometric evaluation the number of glial cells per certain area of the slice (NC – number of cells) was measured. The measurement was performed in ImageJ 1.50 (NIH, Bethesda, Maryland, United States, freeware). Acquired data were processed in the software environment SPSS Statistics (version 18.0, SPSS, Chicago, Illinois, USA) for statistical evaluation. To determine the nature of the sample distribution, Shapiro-Wilk test for normality was performed. Statistical analysis involved the calculation of mean values, standard deviation and mean error. The assessment of the reliability of discrepancies between the studied indicators was based on the use of ANNOVA test with following pared parametric Student's t-test. Statistical discrepancies were considered significant at a confidence level of 95 percent (P < 0.05).
RESULTS
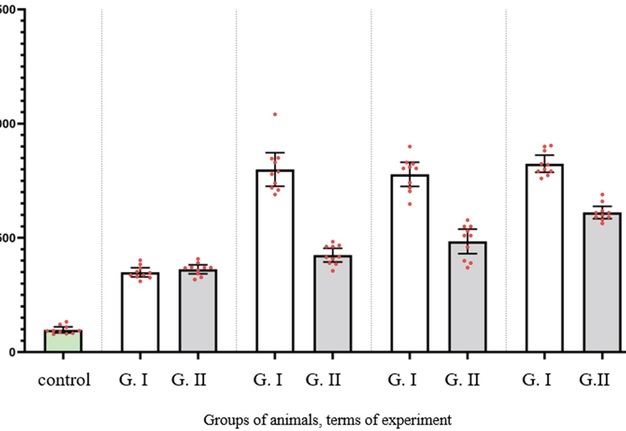
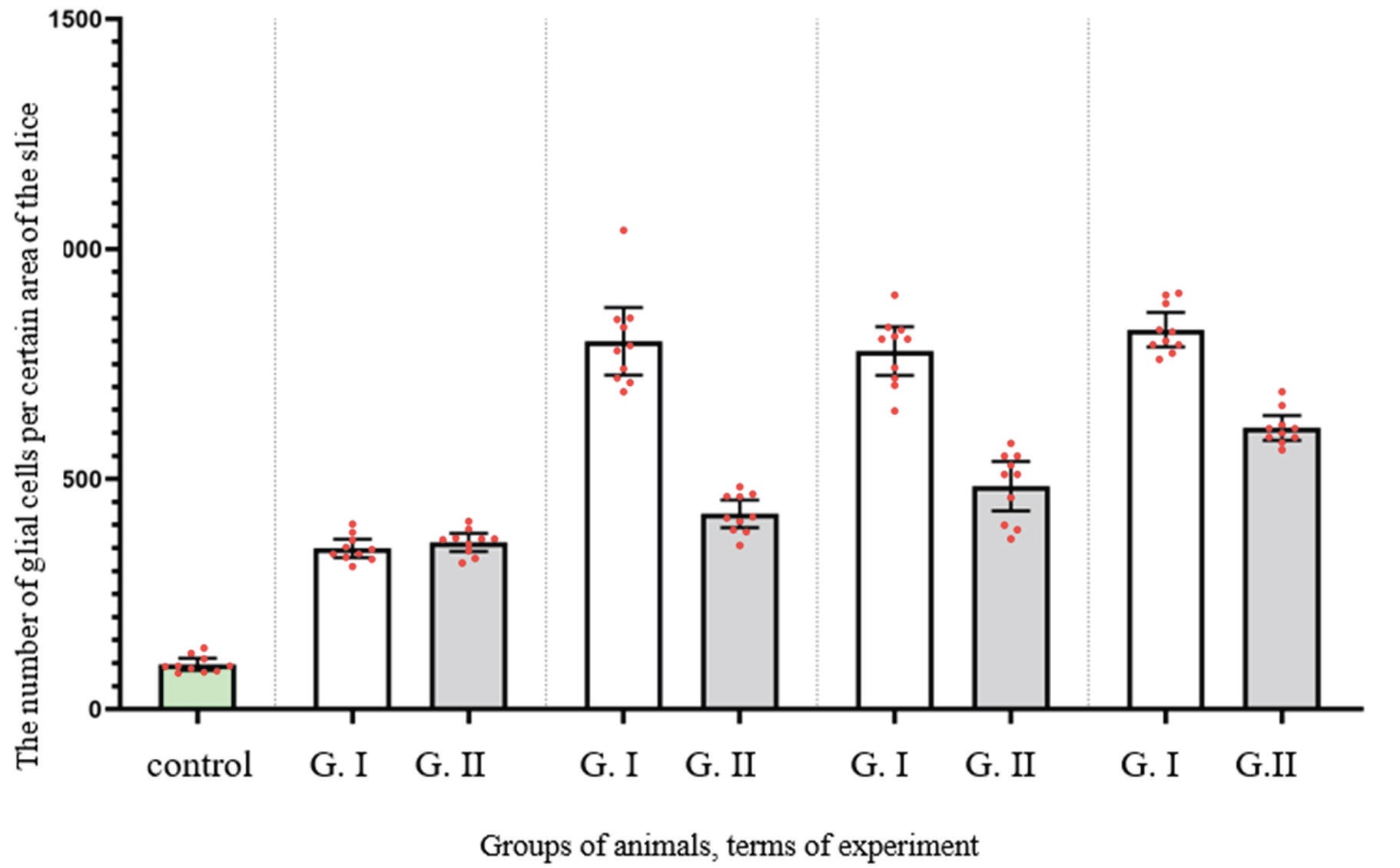
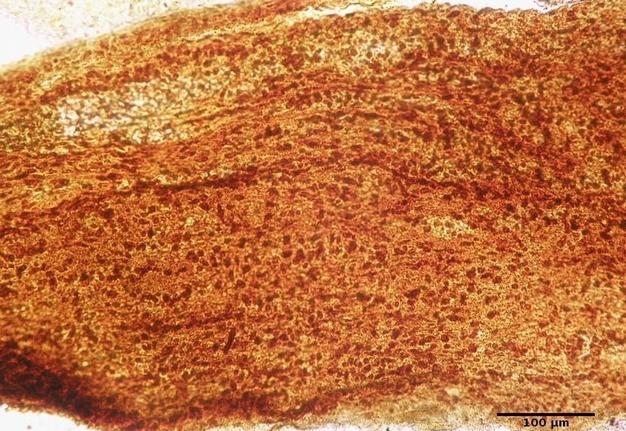
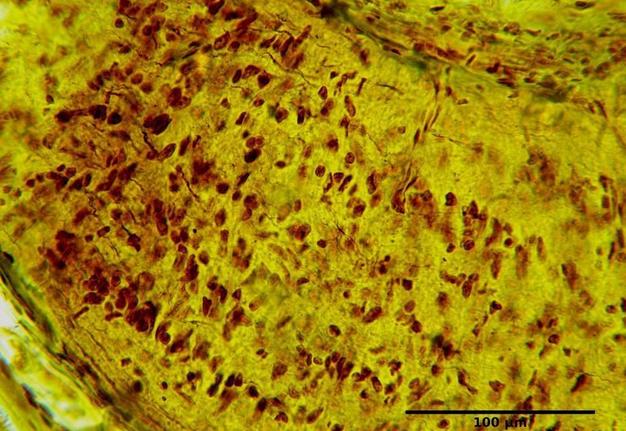
Due to pared comparison of groups, it was noted that the NC in all groups and at all terms of experiment were significantly higher, comparing with control (P < 0.0001 for all comparisons) (Fig 1).
Common to animals of both experimental groups was the development of early degenerative changes in response to injury. Such changes were manifested not only by the development of retrograde, but also by anterograde degeneration of nerve fibers at the observation period of 3 and 6 weeks. The general trend in animals of both groups was a slower course of an anterograde degeneration than of a retrograde one. Differences were also found between animals of experimental Groups I and II: at 3 weeks the degeneration process was faster and more complete in Group II animals, which was manifested in less amount of myelin and less number of cells in all parts of the nerve.
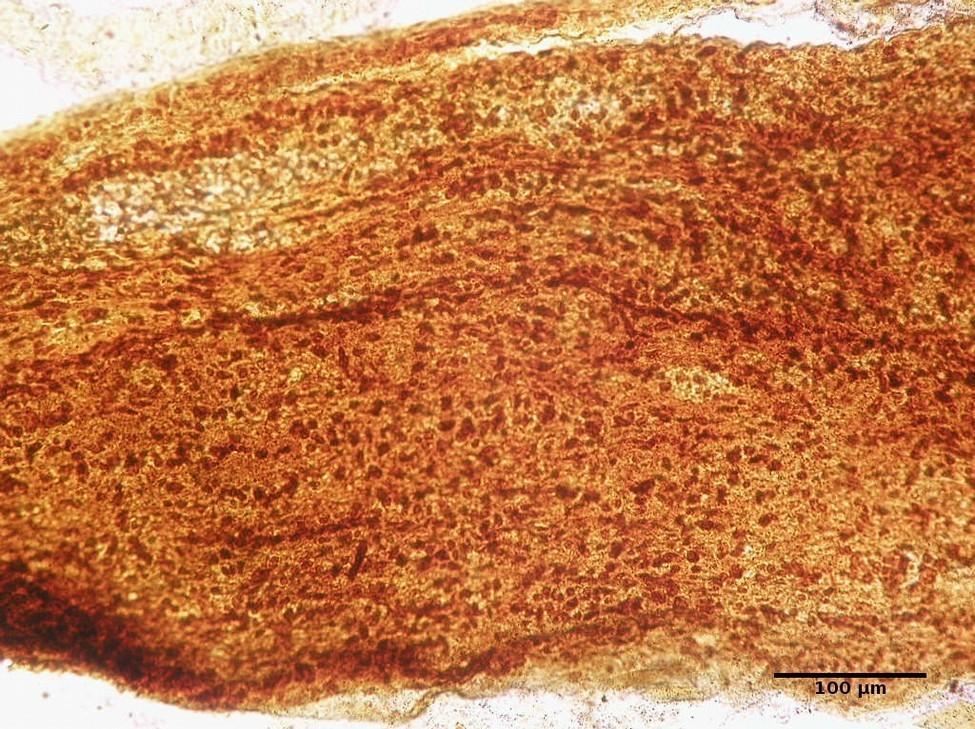
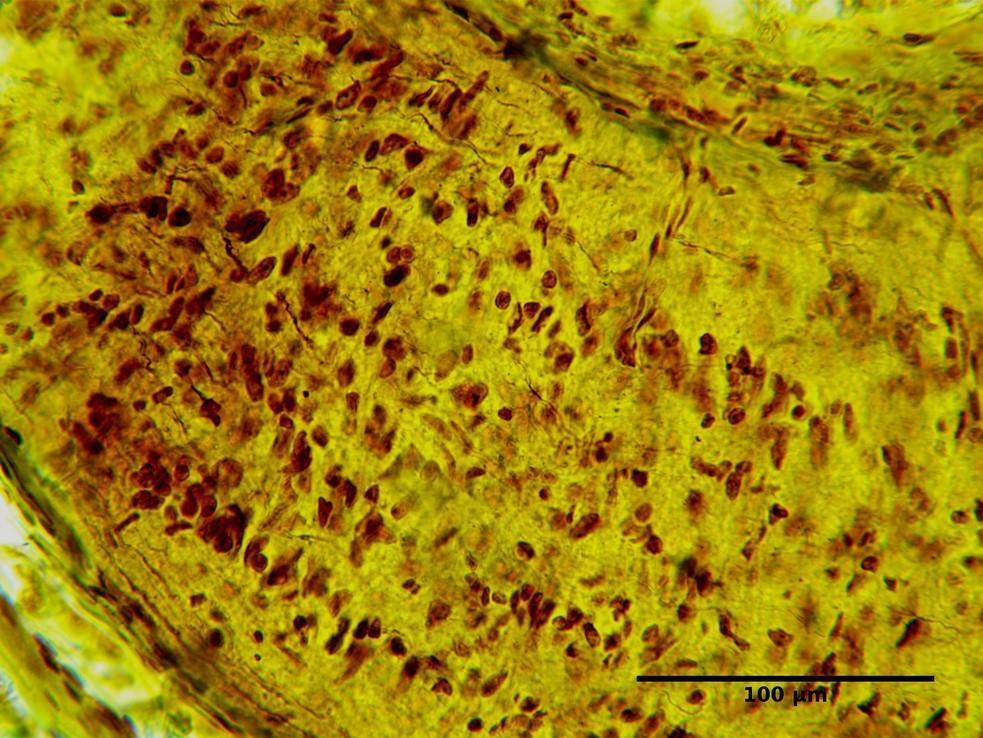
Comparing the NC between Group І and control after 3 week of observation (Fig 2) it was higher in Group I more than 258.8% (P < 0.0001). The same tendency was noted in Group II in the same terms of observation (Fig 3): the NC was 272.4% higher than in control (P < 0.0001).
After 6 weeks of observation in animals of both experimental groups the changes indicated both the completion of degeneration and the beginning of regeneration. However, animals of Group II demonstrated more intensive regeneration processes and earlier onset, what was probably due to faster decay of degraded myelin and is manifested by more nerve fibers in the central part of the nerve, fewer astrocytes and a more orderly arrangement. The NC in Group I decreased and became much higher than at previous terms: more then 128.9% (P < 0.0001). At the same time, the NC in Group I have not risen so dramatically and was higher comparing with previous terms only 17.1% (P = 0.0212).
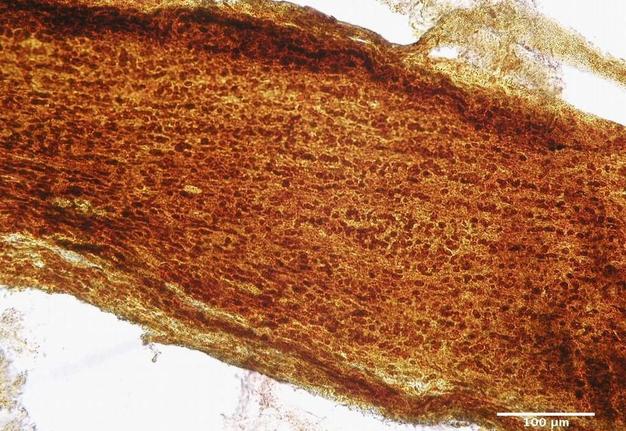
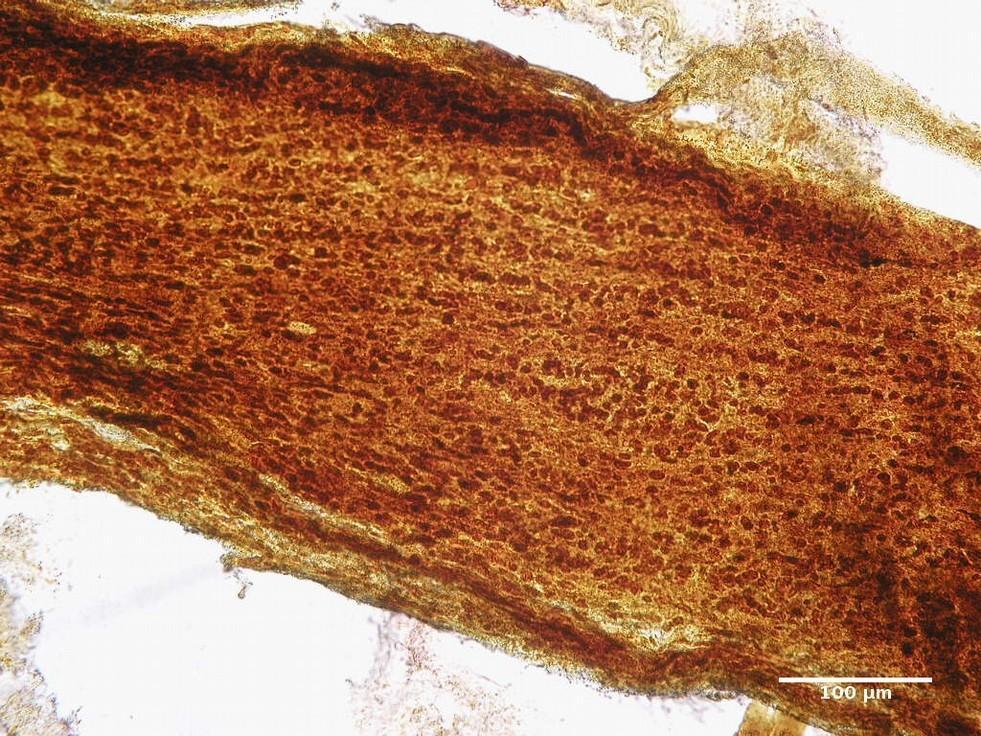
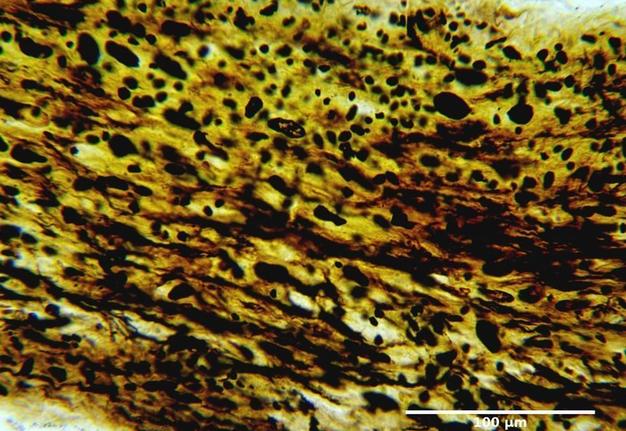
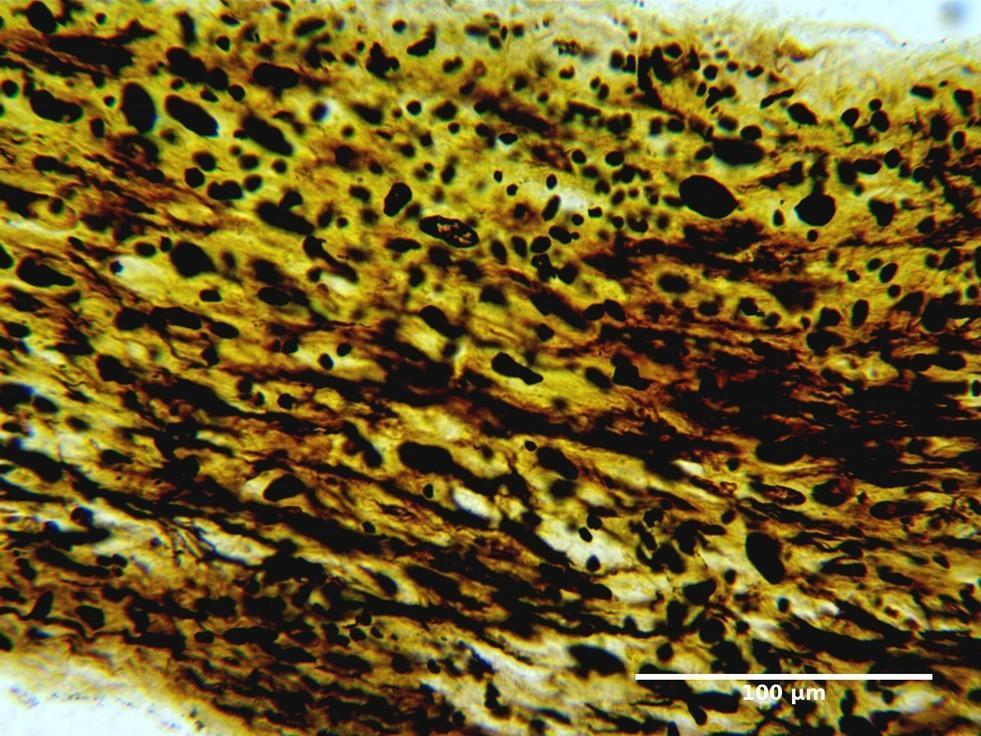
The changes noted at 12 weeks (Figs 4 and 5) after injury indicated a more pronounced and complete regeneration of the nerve fibers, which was manifested in greater numbers in all parts of the optic nerve in animals of Group II. Also, a less number of astrocytes in all parts of the optic nerve in animals of Group II was observed, which can be regarded as less pronounced signs of glial scarring in animals of this group.
Between the animals of Group II at terms of 12 and 24 weeks, the NC was high and did not differ significantly between this terms of observation (ANOVA P = 0.4379). In contrast, the NC in Group II stopped rising between 6 and 12 weeks demonstrating statistical equality (P = 0.4563).
The changes detected at 24 weeks were typical for late neural regeneration. In animals of Group I, regeneration was defective, manifested only by single fibers that have sprouted through the site of injury, which contained a large number of cells and could be regarded as the formation of a glial scar. The decrease in the number of nerve fibers in Group II animals at this observation period could probably be explained by the degeneration of those nerve fibers that have not reached the end point of innervation. At this terms there were no significant differences in Group I comparing with results, noted after 12 weeks. At the same time, Group II demonstrated increase of the NC in 26.1%, comparing with previous term of observation (P = 0.0059).
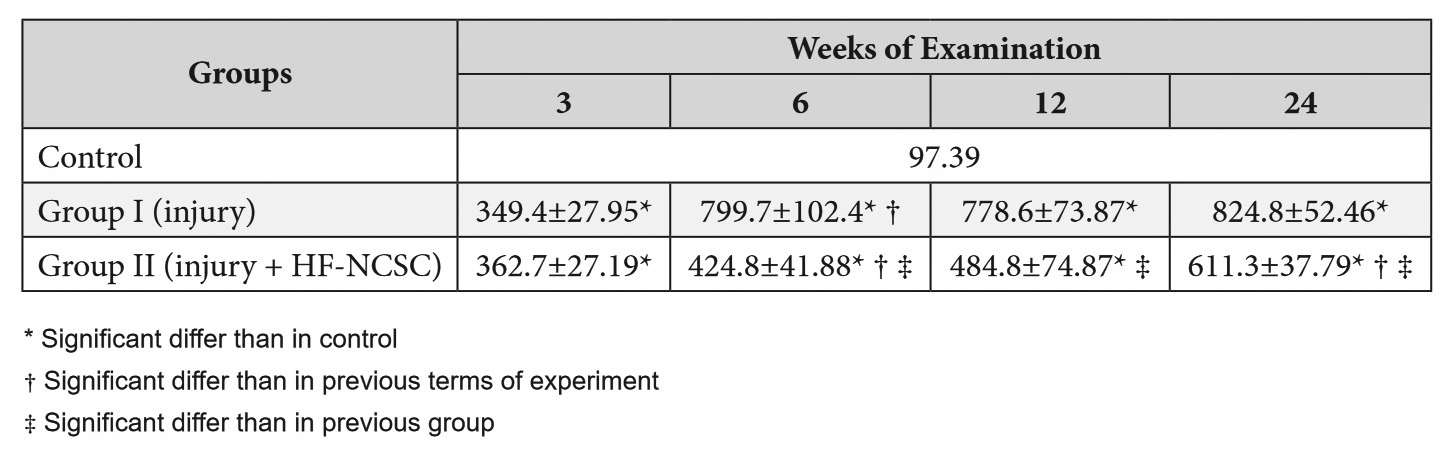
Comparing both groups, the NC was statistically equal only after two weeks of experiment (P = 0.9923). However, after 6 weeks, it was lower than 46.9 % in Group II (P < 0.0001). The same tendency was noted at the terms of 12 and 24 weeks, when it was lower more then 37.7% and 25.9% respectively (P < 0.0001 for both terms) (Table 1).
TABLE 1. Number of Glial Cells per Certain Area of the Slice in Different Terms of the Experiment
DISCUSSION
The developed model of orbital trauma of the orbital allows reproducing destructive changes in its content and deep degeneration of the optic nerve, which is consistent with the literature.11,1,7 According to the literature there are several major factors that complicate the regeneration of the optic nerve. On the one hand, trauma induces apoptosis of retinal ganglion neurons and there is a genetically determined weak ability of injured axons of mature retinal ganglion cells of the retina to regenerate. On the other hand, slow elimination of destructured myelin and formation of glial scar at the site of injury delay the growth of regenerating axons.5 Additionally, there is a difficulty of directed growth
and restoration of initial connections with the brain during regeneration of injured nerve fibres.16
According to the literature, NCSCs have properties that can be used to achieve the goal of the study and can be applied to stimulation of the optic nerve regeneration. The most important of them are ability to multilineage differentiation to glial cells, as well as indirect influence to regeneration due to homing, excretion of vasculogenic or other trophic factors, cytokines and cellular messengers. Numerous studies have proven the survival of stem cells at the site of spinal cord injury up to 6 months after implantation and their low ability to migrate and the lack of tumorigenic potential.16,20–22 It has also been shown that one third of the injected stem cells begin to express RIP (marker of oligodendrocytes), which indicates their differentiation into immature oligodendrocytes. The other part of the stem cells expresses bIII-Tubulin, which indicates their differentiation into neurons.It was found that NCSCs at the site of injury begin to express VEGF-A and VEGF-B, which contribute to the vascularization of the site of injury.8,12 Analysis of the genetic profile of eNCSCs revealed that these cells express NGF and BDNF (growth factors), which maintain the viability of neurons. It was also found that the axons in the white matter of the injured spinal cord grow towards the implanted eNCSCs.23
According to our study, the use of stem cells initiates the acceleration and improvement of the regeneration of the optic nerve, as evidenced by the appearance of young newly formed nerve fibers, glial cell columns, which are formed mainly of oligodendrocytes, reducing glial scarring by reducing the number of astrocytes and remnants of destroyed myelin. The presence of nerve fibers in the second group of animals at week 6 of the study in the central and peripheral segments can be explained by the ability of stem cells to protect retinal ganglion neurons and the ability of eNCSCs to transform into neurons. The appearance of more ordered columns of oligodendrocytes can be attributed to the ability of stem cells to differentiate in the direction of immature oligodendrocytes. An increase in the number of oligodendrocytes and a decrease in the number of astrocytes may reduce the severity of the glial scar at the site of injury. Acceleration of elimination of the destroyed myelin remains is possible due to improvement of vascularization of the injured nerve that is caused by ability of stem cells to express the corresponding factors.
The changes detected after 12 and 24 weeks of the study, in the form of a decrease in the number of nerve fibers in group I can be regarded both as a delay in regeneration and as a consequence of its inferiority, because of an increase of glial cell number. According to studies, conducted by Fitzgerald et al4 and Zhang et al24, an increase of glial cell number is a universal response to trauma and axonal degeneration or death of retinal ganglion cells. In contrast, in group II animals after 12 and 24 weeks, although there was a decrease in the number of nerve fibers in the peripheral segment, the growth of the gliocytes number was not so rapid, indicating less pronounced scarring and more successful regeneration.
CONCLUSIONS
It can be assumed that the application of mesenchymal stem cells, namely derivates of the neural crest, after the experimental orbital trauma, stimulates a recovery of the optic nerve. Further studies should be performed to study more deeply the neural crest derived stem cell populations, involved into recovery of damaged optic nerves.
FUNDING
This study did not receive any funding source.
ROLE OF CO-AUTHORS IN WRITING
All authors contributed equally to the concept and design of the study; writing, editing, and final review of the manuscript.
REFERENCES
- Liu B, Hunter D, Smith A, Chen S, Helms JA. The capacity of neural crest-derived stem cells for ocular repair. Birth Defects Res C Embryo Today 2014;102(3):299–308. Crossref | Medline | Google Scholar
- Maclaren R. Regeneration and transplantation of the optic nerve: developing a clinical strategy. Br J Ophthalmol 1998;82(5):577–83. Crossref | Medline | Google Scholar
- Chepurnyi Y, Chernogorskyi D, Kopchak A, Petrenko O. Clinical efficacy of peek patient-specific implants in orbital reconstruction. J Oral Biol Craniofac Res 2020;10(2):49–53. Crossref | Medline | Google Scholar
- Fitzgerald M, Bartlett CA, Harvey AR, Dunlop SA. Early events of secondary degeneration after partial optic nerve transection: an immunohistochemical study. J Neurotrama 2010;27(2):439–52. Crossref | Google Scholar
- Gall C, Lucklum J, Sabel BA, Franke GH. Vision- and health-related quality of life in patients with visual field loss after postchiasmatic lesions. Invest Ophthalmol Vis Sci 2009;50(6):2765–76. Crossref | Google Scholar
- Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 2014;20(8):857–69. Crossref | Google Scholar
- Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, Miller FD, Tetzlaff W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci 2007;27(36):9545–59. Crossref | Google Scholar
- Sieber-Blum M, Schnell L, Grim M, Hu YF, Schneider R, Schwab ME. Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Mol Cell Neurosci 2006;32(1-2):67–81. Crossref | Google Scholar
- Greenwald B, Kapoor N, Singh A. Visual impairments in the first year after traumatic brain injury. Brain Inj 2012;26(11):1338–59. Crossref | Google Scholar
- Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, Schaffer DV, Flannery JG, Schwab ME. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis 2013;51:202–13. Crossref | Google Scholar
- Kordium V, Chaikovsky Yu, Irodov D, Drahulian M, et al. Modelling of systemic lesion of organism for development of multitarget cellular and cytokine therapy. Biopolym Cell 2016;32(5):381–94. Crossref | Google Scholar
- Sieber-Blum M. Epidermal neural crest stem cells and their use in mouse models of spinal cord injury. Brain Res Bull 2010;83(5):189–93. Crossref | Google Scholar
- Blentic A, Tandon P, Payton S, Walshe J, Carney T, Kelsh RN, Mason I, Graham A. The emergence of ectomesenchyme. Dev Dyn 2008;237(3):592–601. Crossref | Google Scholar
- Dupin E, Coelho-Aguiar J. Isolation and differentiation properties of neural crest stem cells. Cytometry A 2013;83(1):38–47. Crossref | Google Scholar
- Kaltschmidt B, Kaltschmidt C, Widera D. Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Rev Rep 2012;8(3):658–71. Crossref | Google Scholar
- Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn 2004;231(2):258–69. Crossref | Google Scholar
- Chepurnyi YV, Kustrjo TV, Korsak AV, Likhodievskyi VV, Rodnichenko AE, Gubar OS, Zlatska OV, Kopchak AV, Zabila AO, Olefir SS, Zubov DO, Vasyliev RG, Chaikovskyi YB. Influence of postnatal multipotent stem cells (derived from neural crest) on regeneration of orbital soft tissue content after experimental injury. Probl Cryobiol Cryomed 2018;28(1):59–63. Crossref | Google Scholar
- Vasyliev R., Rodnichenko A., Shamalo S, Demidchouk AS, Labunets IF, Chaikovskii YuB, Butenko GM. Effects of neural crest-derived multipotent stem cells on regeneration of an injured peripheral nerve in mice. Neurophysiology 2015;47(1):80–3. Crossref | Google Scholar
- Kolomiitsev A, Chaikovsky Yu, Tereschenko T. Rapid method for impregnation of the components of the peripheral nervous system with silver nitrate [in Russian]. Arkh Anat Gistol Embriol 1981;81(8):93–6. Google Scholar
- Wender M, Adamczewska-Goncerzewicz Z, Goncerzewicz A. Myelin lipids in Wallerian degeneration of the rabbit optic nerve. Exp Pathol (Jena) 1979;17(6):334–9. Crossref | Google Scholar
- Watanabe M. Regeneration of optic nerve fibers of adult mammals. Dev Growth Differ 2010;52(7):567–76. Crossref | Google Scholar
- Yu F, Zhang R. A novel model of optic nerve injury established by microsurgery using the pterional approach in cats. Neurol India 2011;59(3):355–61. Crossref | Medline | Google Scholar
- Sieber-Blum M, Grim M. The adult hair follicle: cradle for pluripotent neural crest stem cells. Birth Defects Res C Embryo Today 2004;72(2):162–72. Crossref | Google Scholar
- Zhang S, Wang H, Lu Q, Wang N, Wang Y, Yang D, Yan F. Detection of early neuron degeneration and accompanying glial responses in the visual pathway in a rat model of acute intraocular hypertension. Brain Res 2009;1303:131–43. Crossref | Google Scholar