Clinical Efficacy of Patient-specific Implants Manufactured using Direct Metal Laser Sintering (DMLS) Technology in Patients with Mandibular Defects
September 30, 2020
https://doi.org/10.23999/j.dtomp.2020.9.3
J Diagn Treat Oral Maxillofac Pathol 2020;4:162–77.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE
Chernogorskyi DM, Voller MV, Vasilyev AS, Chepurnyi YV, Kopchak AV. Clinical efficacy of patient-specific implants manufactured by direct metal laser sintering (DMLS) technology in patients with
mandibular defects. J Diagn Treat Oral Maxillofac Pathol 2020;4(9):162–77.
Contents: Abstract | Introduction | Materials and methodos | Results | Discussion | Conclusions | Term of consent | Conflict of interest | Role of the authors | Fundings | Acknowledge | References (25)
ABSTRACT
Purpose: The purpose of this study was to compare the clinical efficacy of patient-specific implants (PSIs) in patients with mandibular defects in the early and distant postoperative period.
Materials and Methods: The surgical results in 60 patients with postoperative and posttraumatic mandibular discontinuous defects were analyzed. The patients were treated at the Center of Maxillofacial Surgery and Dentistry, Kyiv Regional Clinical Hospital in the period from 2015 to 2020.
Results: Despite certain functional limitations and residual aesthetic deficiency, 34 patients (85%) of the main group and 9 (45 percent) of the control group noted an improvement in their quality of life and were satisfied with the results of the operation (р < 0.05).
Conclusions: The use of PSIs, compared to traditional methods of bone grafting, allow to achieve a more accurate restoration of the anatomical shape of the mandible in areas with complex geometry and probably better aesthetic results, and significantly reduces the frequency of secondary displacement of bone fragments due to plastic deformation and destruction of fixation elements (p < 0.05). At the same time, it probably does not affect the frequency of purulent-inflammatory complications, unsatisfactory clinical results and the effectiveness of the restoration of masticatory function in patients with mandibular defects.
INTRODUCTION
Treatment of patients with posttraumatic and postoperative mandibular defects is an urgent medical and social problem. Large defects, accompanied by a significant breach of bone continuity, lead to cosmetic deficiency, impaired chewing, swallowing and speech, deterioration of somatic health of severe psycho-emotional disorders and reduced quality of life. 1
The main objectives of comprehensive treatment of such patients are to ensure adequate masticatory function and acceptable aesthetic outcome. 2
To date, a significant number of surgical interventions have been proposed to replace mandibular defects, in particular with the use of bone grafts, endoprostheses, patient-specific implants, tissue engineering methods, and distraction osteogenesis. 3
The gold standard for the treatment of large mandibular defects today is considered to be the use of vascularized and non-vascularized autologous bone transplants from the fibula, iliac crest, scapula,
etc. 4,5 They allow not only to restore the continuity and shape of the mandible, but also to create conditions for future prosthetic rehabilitation with the use of dental implants. 6,7
One of the important problems is the significant discrepancy between the donor bone and intact mandible in its geometric parameters, architecture, mechanical, and biological properties. For functionally stable fixation of bone grafts, preformed reconstructive plates are used, the adaptation of which to the relief of bone fragments of the jaw and graft is performed directly in the wound or on stereolithographic models (Fig 1). 8,9
Depending on the severity of the clinical case, the surgeon's skills and experience, these procedures can take a long time, and their accuracy and effectiveness will be affected by subjective factors.
The existing shortcomings and limitations of traditional bone grafting methods using preformed standard plates have led to the emergence of a new concept – the creation of patient-specific implants
(PSIs). 10,11
They are made on the basis of pre-created virtual design using computer-assisted design/computer-assisted (CAD/CAM) technology. 12,13
Such structures do not require intraoperative bending or shape adaptation and themselves act as a template that determines the correct position of the jaw fragments and bone grafts. 11
The clinical effectiveness of this approach has been demonstrated in the numerous works. 5,7,9,14–16
When using different types of PSI, the authors have demonstrated certain advantages of their use. 17,18
At the same time, the systematic review 19 found no significant differences in the frequency of postoperative complications, length of hospital stay, graft rejection and flap necrosis, as well as the
frequency of secondary surgery with PSI compared to traditional methods of reconstruction. 16
The long-term results of reconstructive surgeries are also insufficiently studied 9 and require additional research and accumulation of clinical material to determine objectively the advantages and disadvantages of this technique, the limits of its use, indications and contraindications for PSI usage, substantiated from the standpoint of evidence-based medicine. 12
The purpose of this study was to compare the clinical efficacy of patient-specific implants in patients with mandibular defects in the early and distant postoperative period.
MATERIALS AND METHODS
The materials of this study included 60 patients with postoperative and posttraumatic mandibular discontinuous defects, who were treated at the Center of Maxillofacial Surgery and Dentistry, Kyiv Regional Clinical Hospital in the period from 2015 to 2020.
The study ensured compliance with the principles of bioethics and patient rights in accordance with the Helsinki Declaration and the Fundamentals of Health Legislation of Ukraine (1992). Examination of the work materials was conducted by the commission on bioethics of Bogomolets National Medical University (protocol #107 dated December 29, 2017).
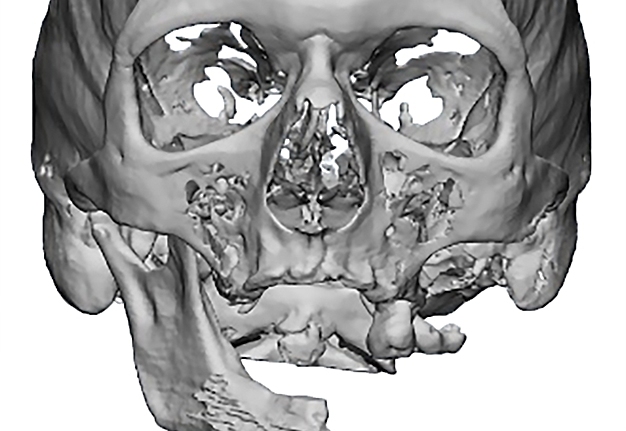
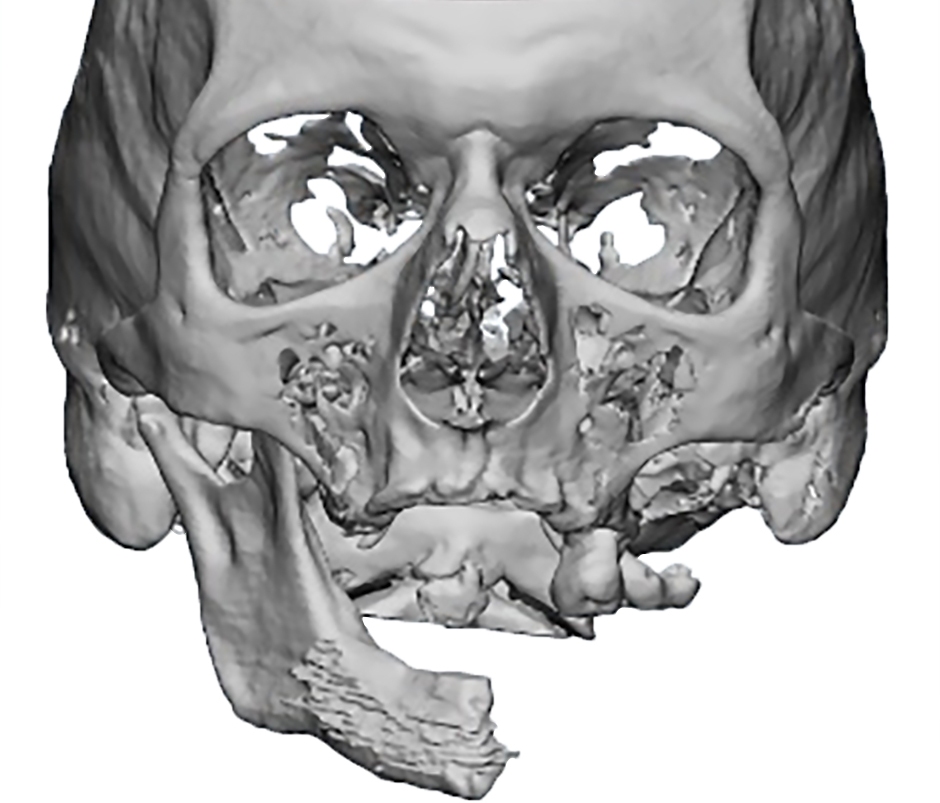
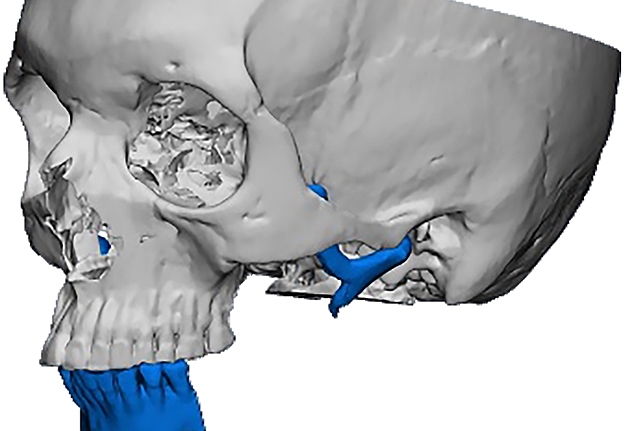
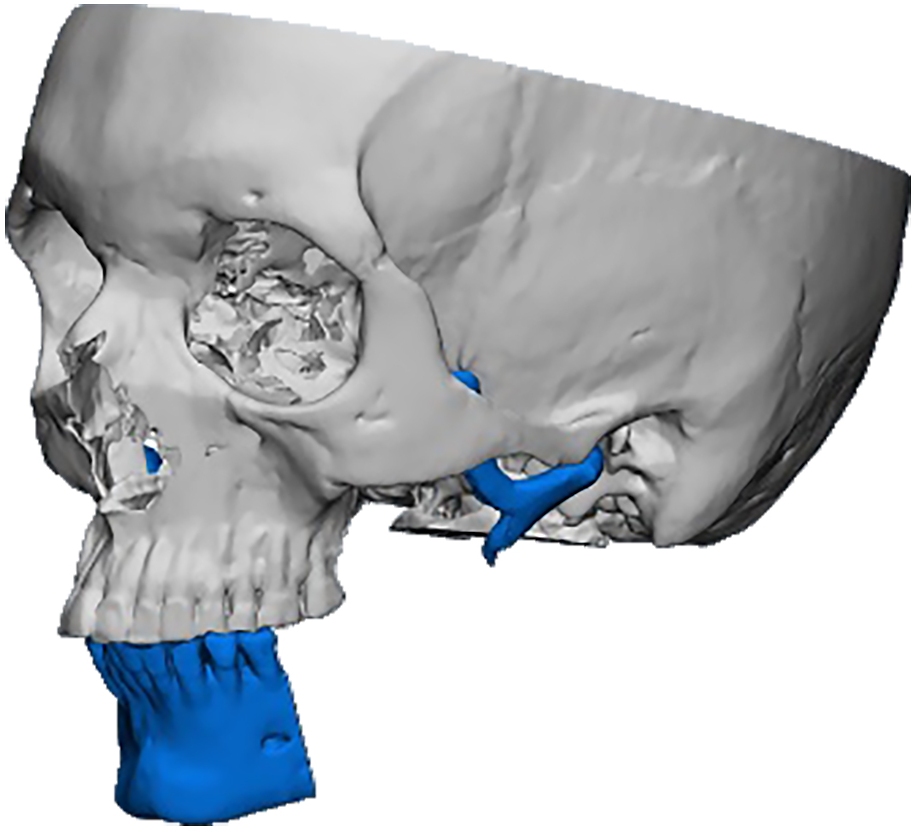
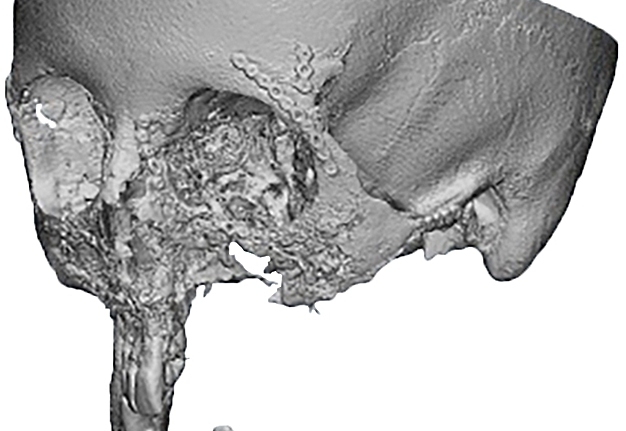
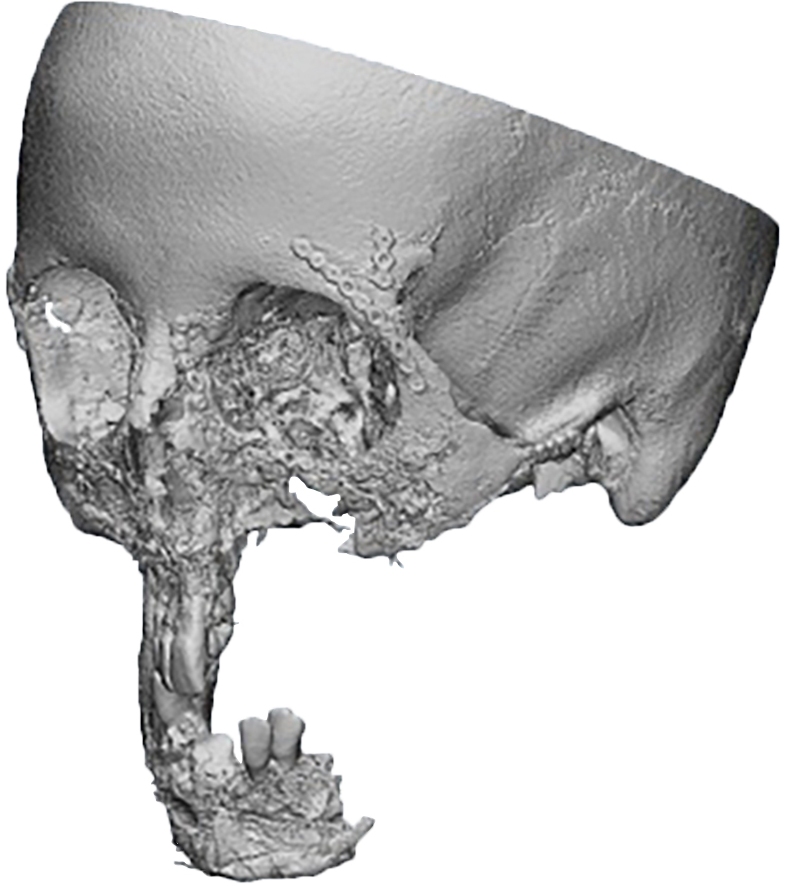
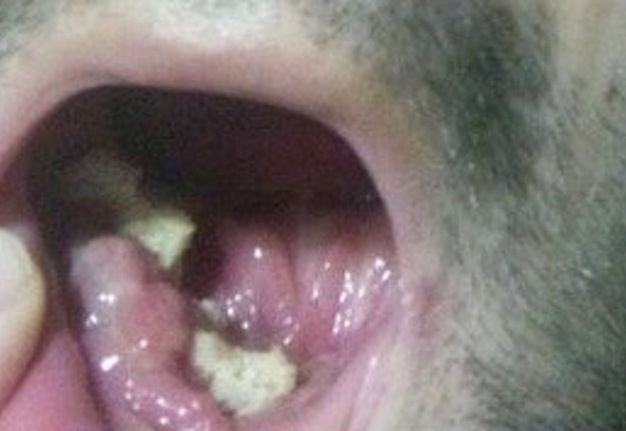
Criteria for inclusion in the study groups were: mandibular discontinuous defects (Fig 2), which required reconstruction in the presence of written informed consent of the patient to participate in the study.
Exclusion criteria were – age up to 16 years, the presence of concomitant somatic pathology in a compensated or a decompensated state, mental illness, chronic alcoholism or drug addiction, active radio- or chemotherapy, non-compliance with medical recommendations and lack of interaction with the doctor, total mandibular defects, incomplete clinical and computed tomography documentation, follow-up period less than 6 months, and patient`s refusal to participate in the study.
Among the patients included in the study, men accounted for 40% and women – for 60 percent.
The age of patients ranged from 16 to 82 years, and averaged 40.9 ± 14.6 years.
All patients were divided into 2 groups, homogeneous in age, sex, severity andetiology of the defect. In the main group (40 patients) the PSI, made by the technology of selective lasersintering of titanium, was used for the replacement of defects.
In the control group (20 patients), traditional methods of replacement of mandibular defects with autologous bone grafts were used in combination with preformed reconstructive plates.
Patients of both groups were examined according to a standard protocol, which included history taking, assessment of general and local status, computed tomography of the facial skeleton, followed by diagnosis and treatment plan. Virtual simulation of surgical interventions and design of PSI was performed by multi-slice computed tomography (MSCT) data on the basis of the Laboratory of Computer Modeling and Digital Dentistry, Bogomolets Dental Medical Center. The location, size and type of defect according to the recommendations were divided into anterior defect (from canine to canine), distal parts of the bodies (in the area of molars and premolars), and defects in areas of two rami.
The design and manufacture of PSI in both groups were based on a standard digital protocol, which provided the following: tomographic data presented as a series of Digital Imaging and Communications in Medicine (DICOM) files were imported into the D2P TM software environment (version 1.0.253, DICOM to PRINT, 3D Systems, Rock Hill, SC, USA).
To create a three-dimensional virtual model of the bones of the facial skull, image segmentation was performed according to the radiological density of tissues, followed by the creation of virtual models of mandibular fragments.
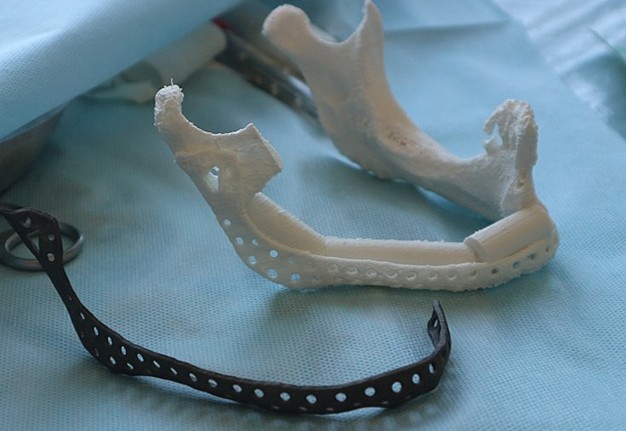
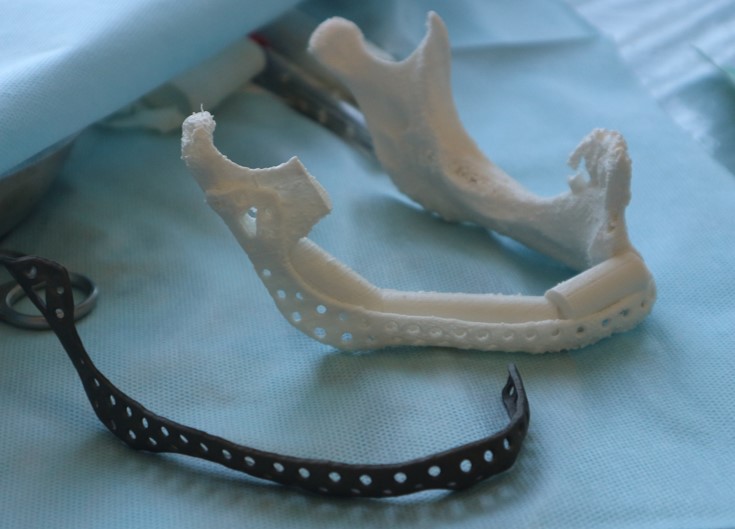
In the main group, virtual models were exported to the software environment Geomagic Freeform Plus TM (3D Systems, Rock Hill, SC, USA) where virtual repositioning of fragments and replacement
of defect with the subsequent creation of design of PSI according to a clinical task were carried out (Fig 3).
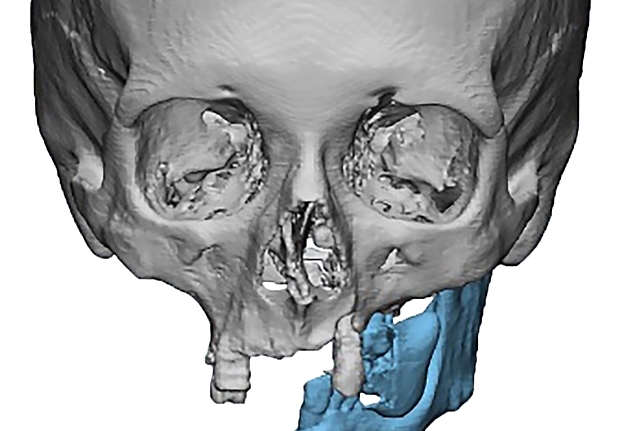
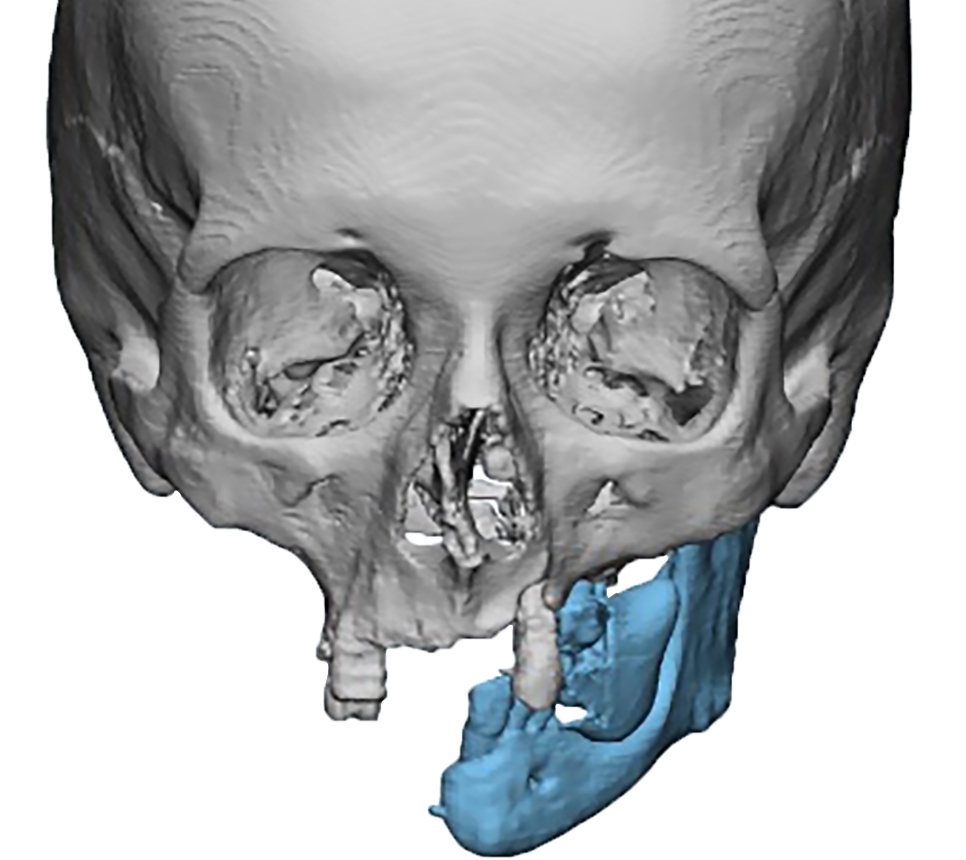
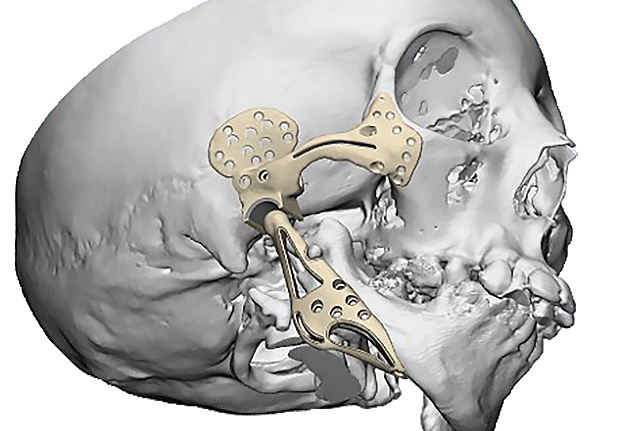
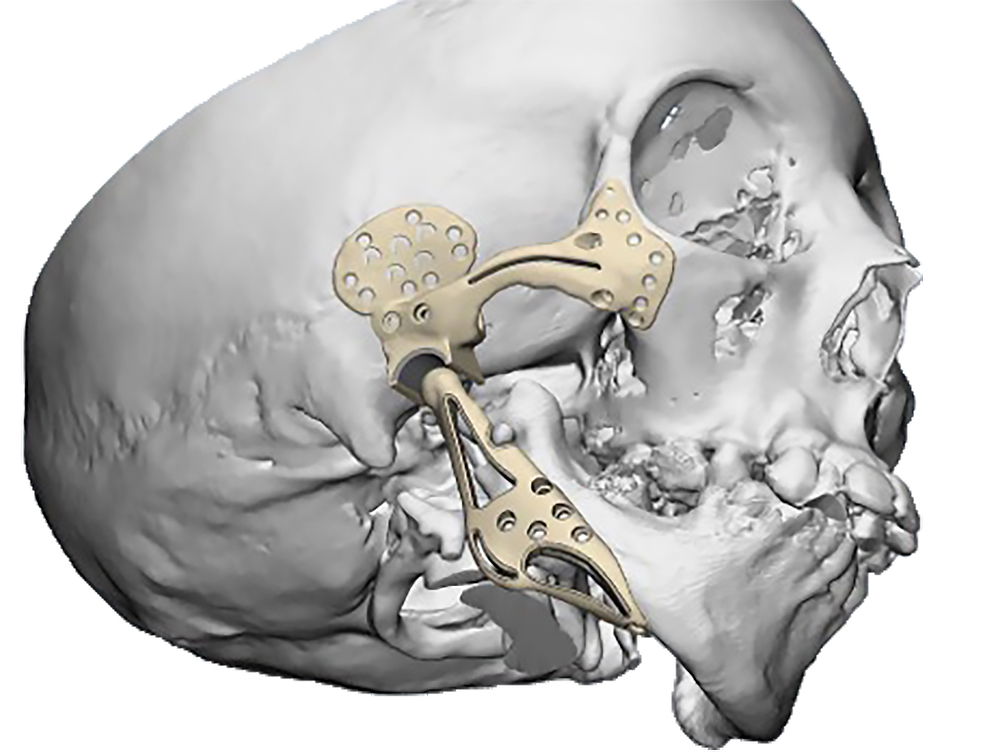
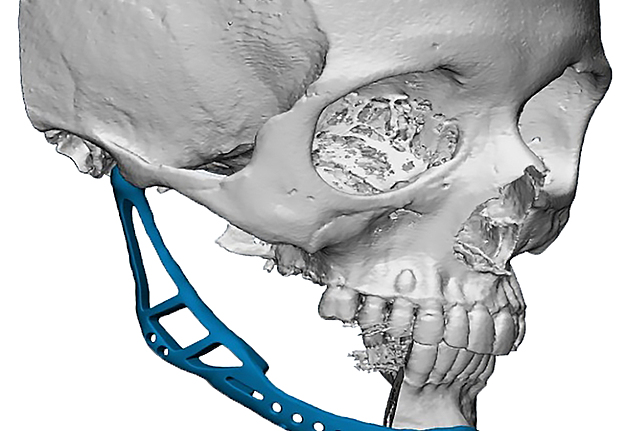
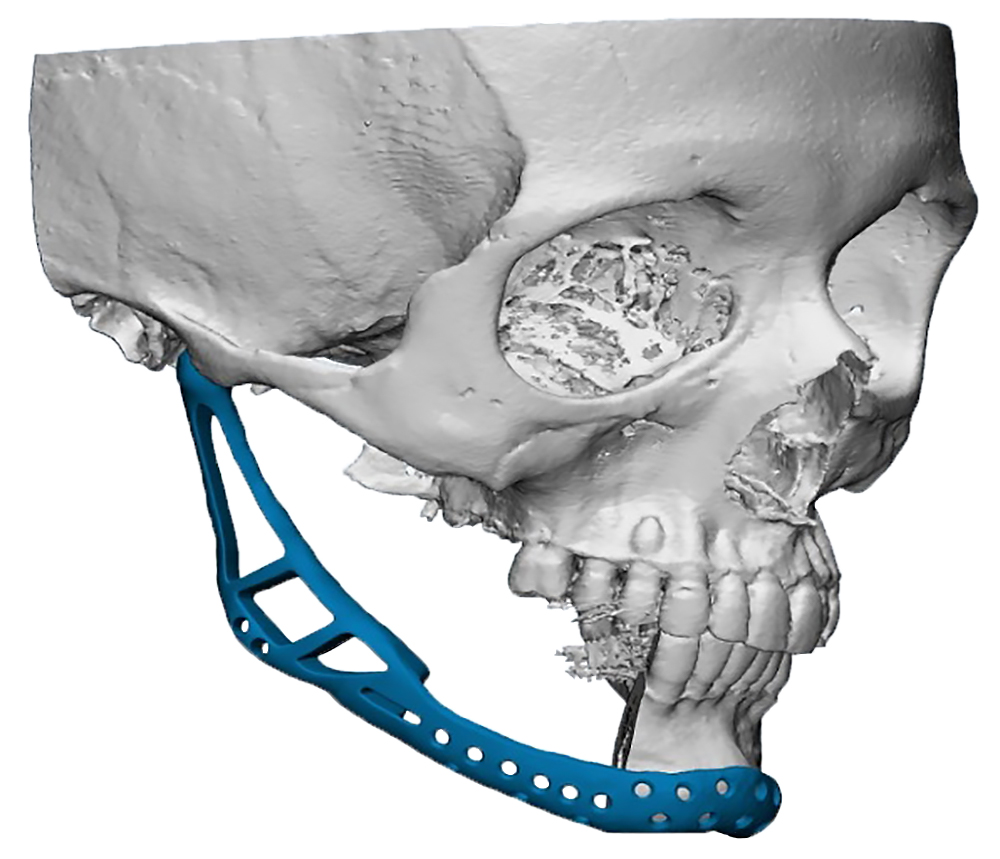
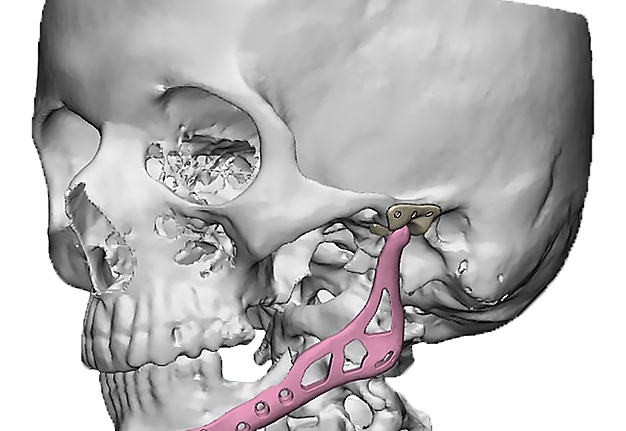
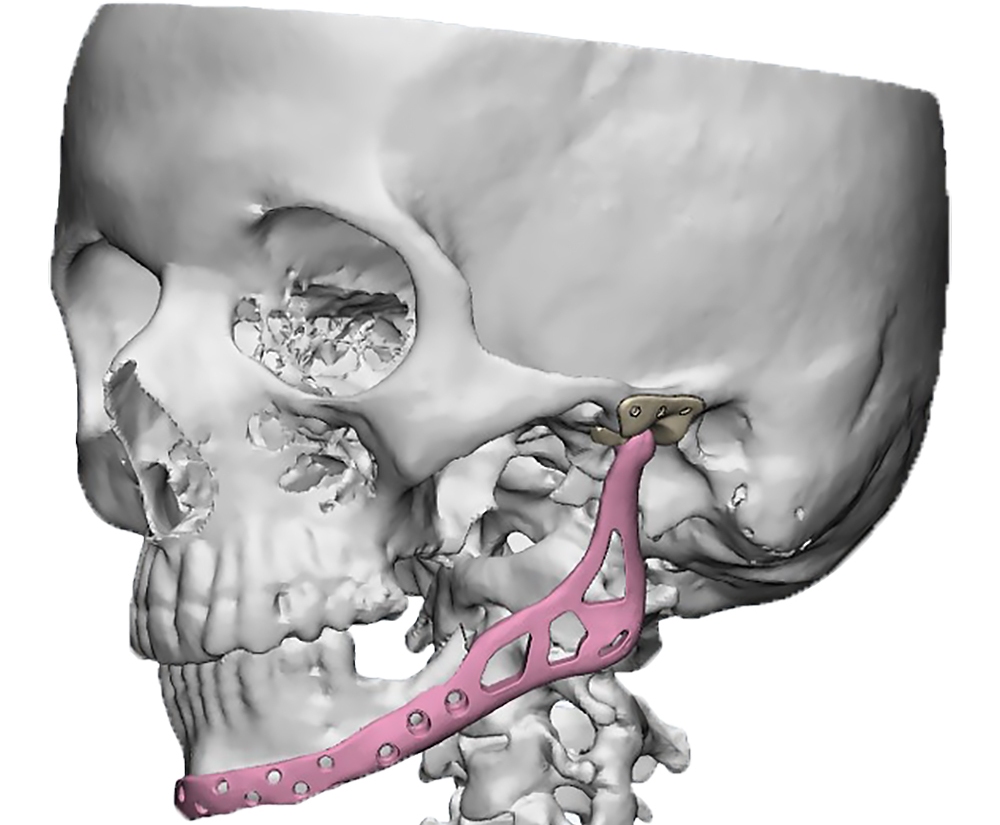
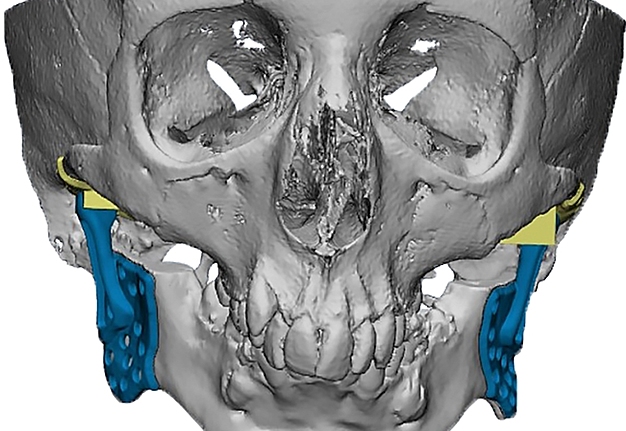
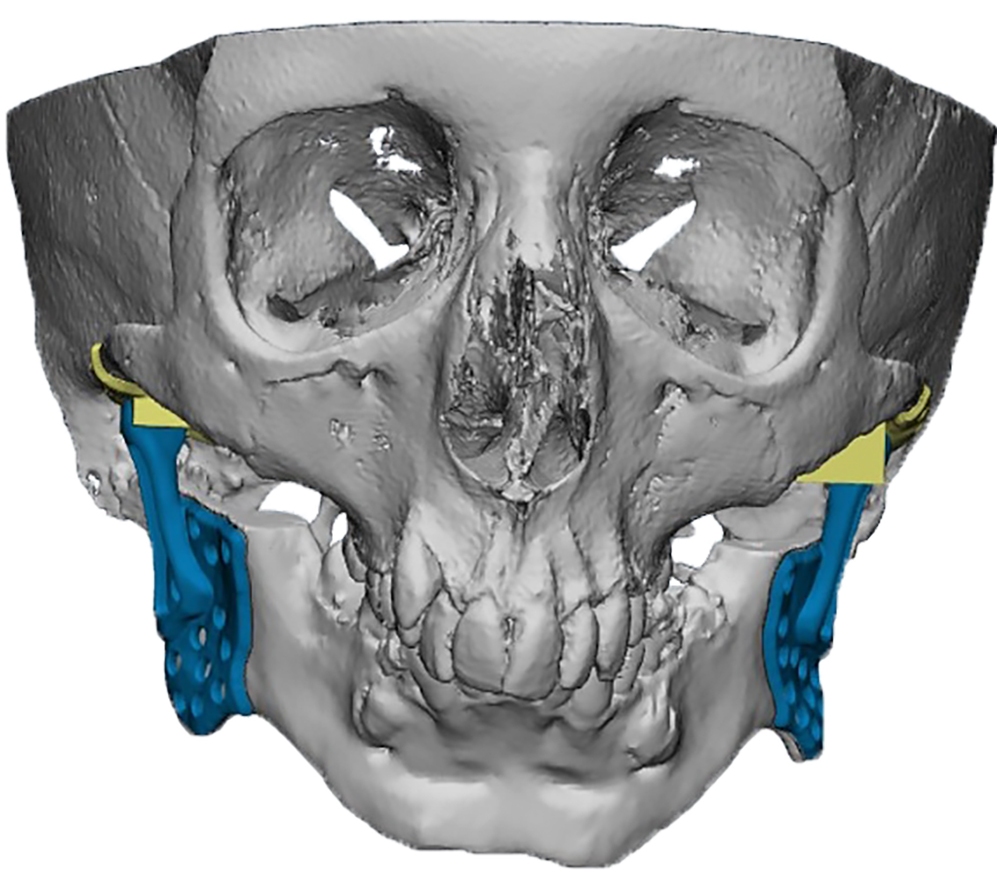
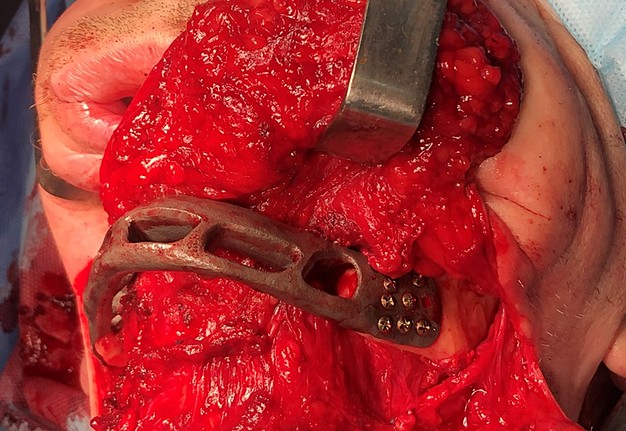
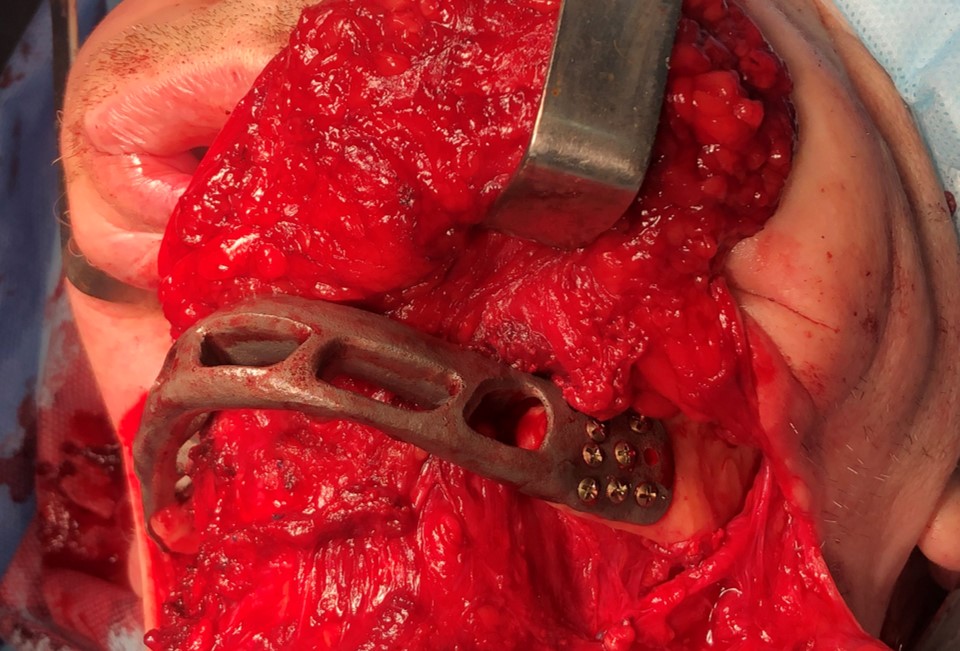
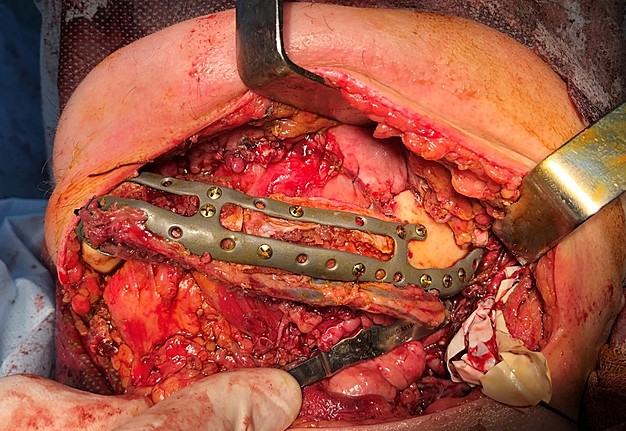
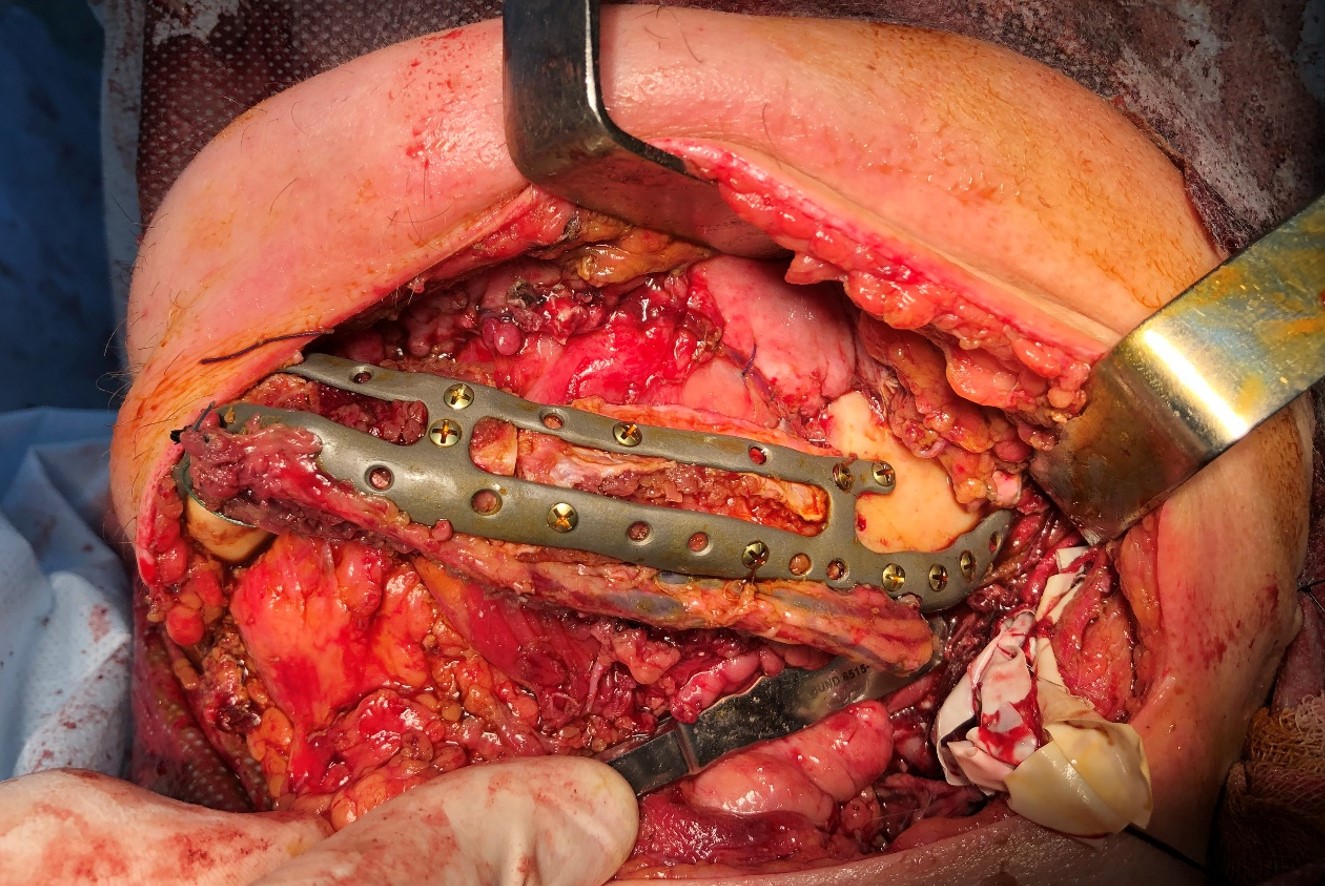
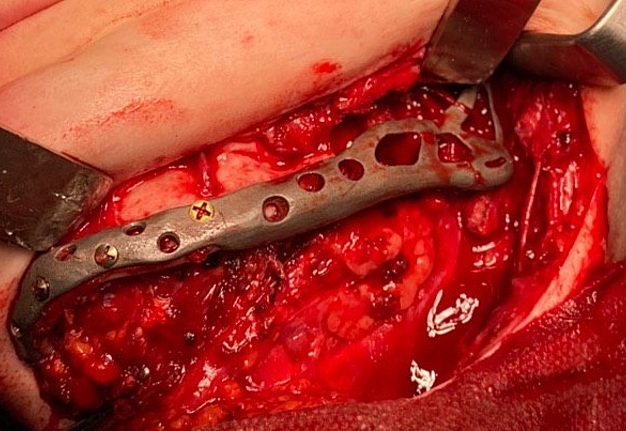
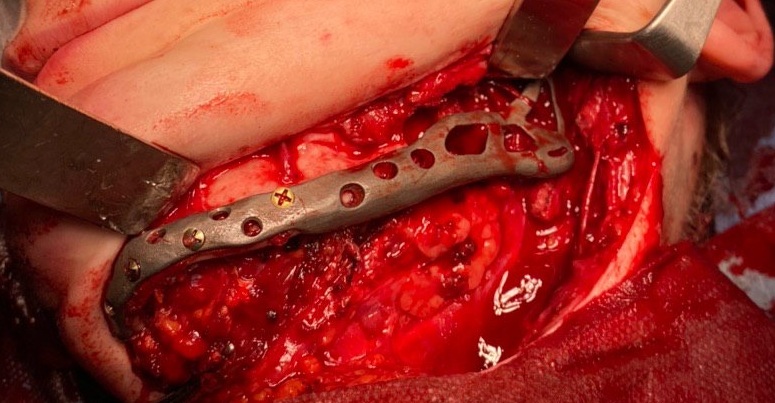
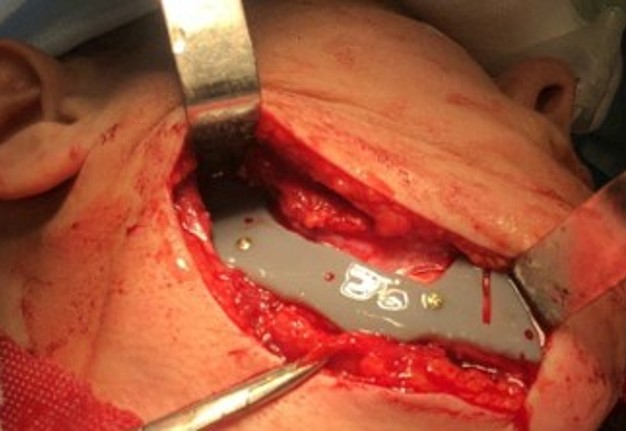
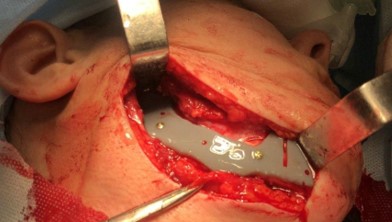
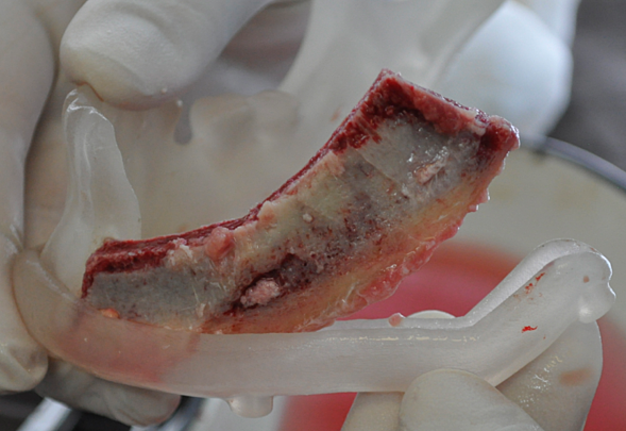
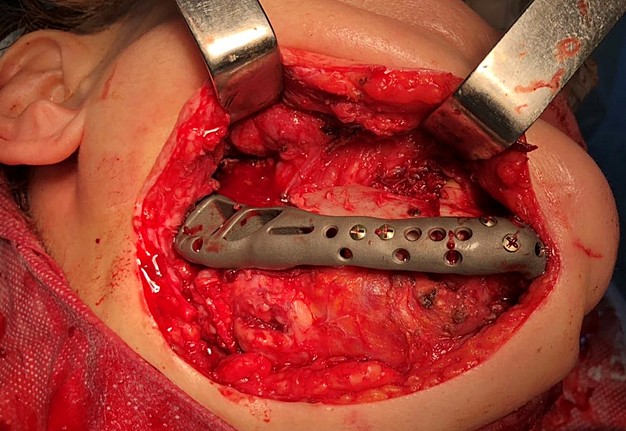
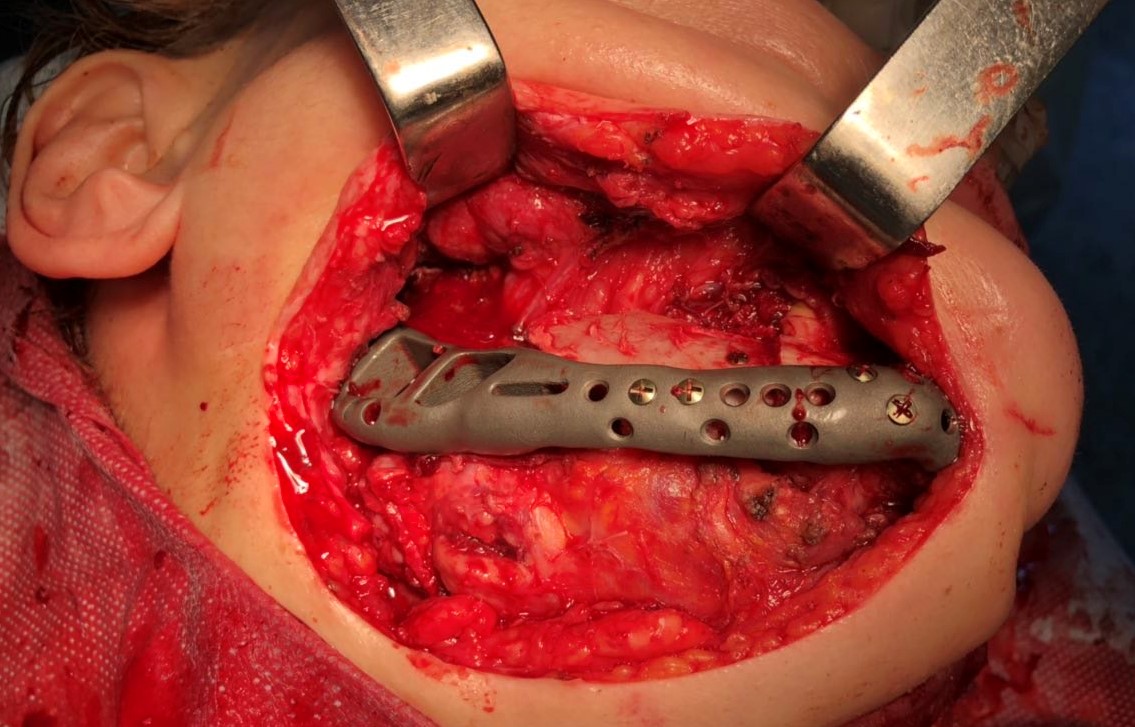
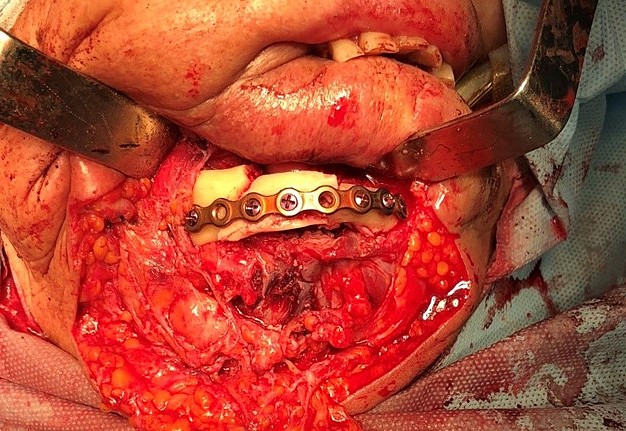
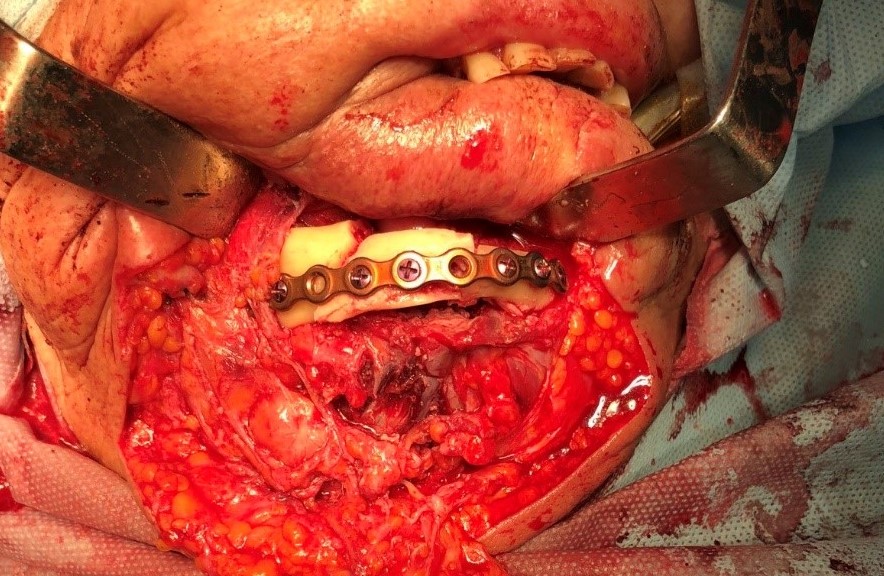
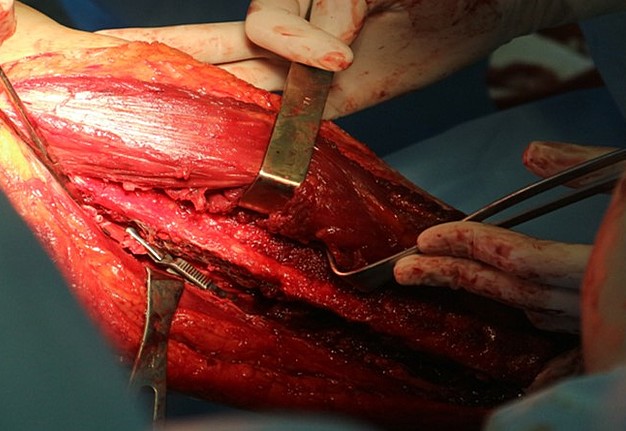
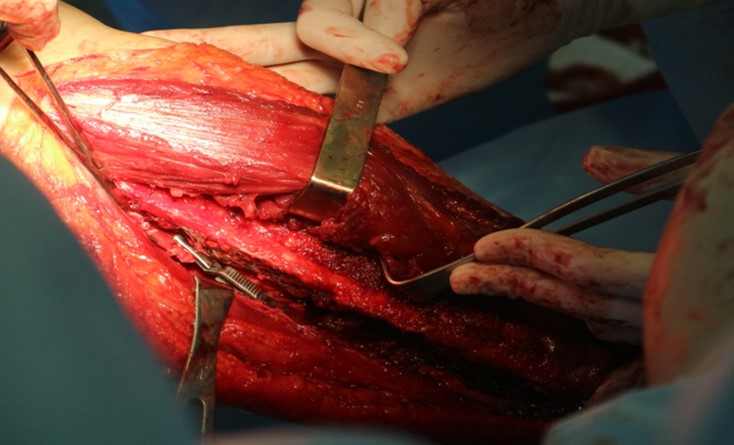
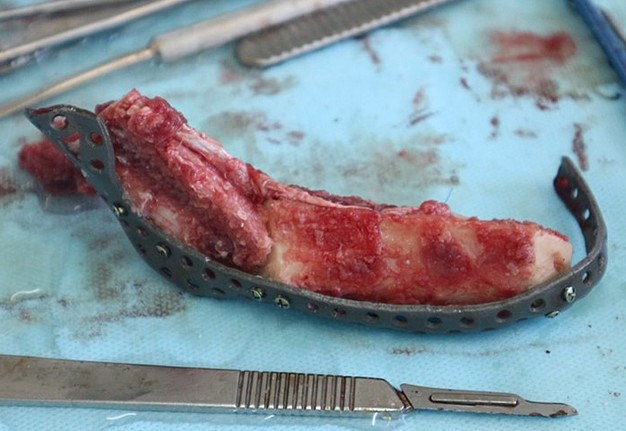
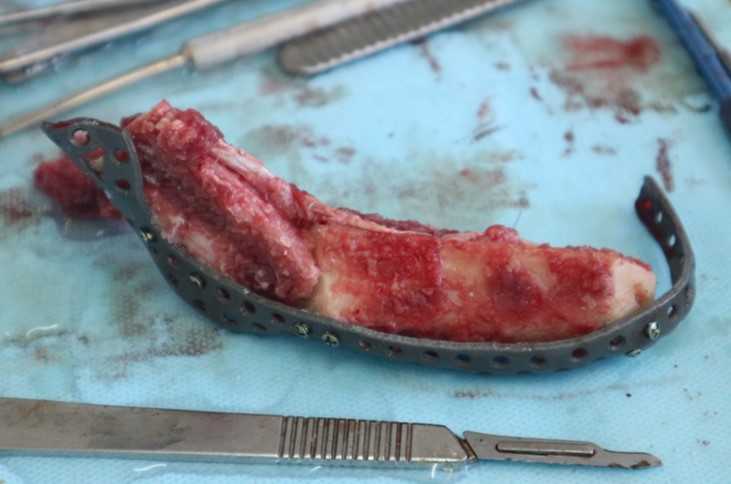
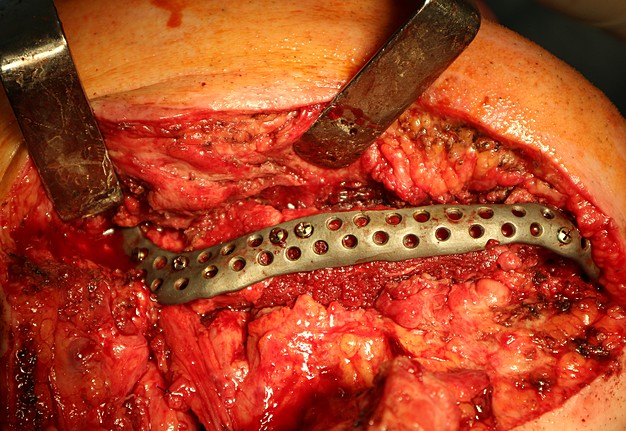
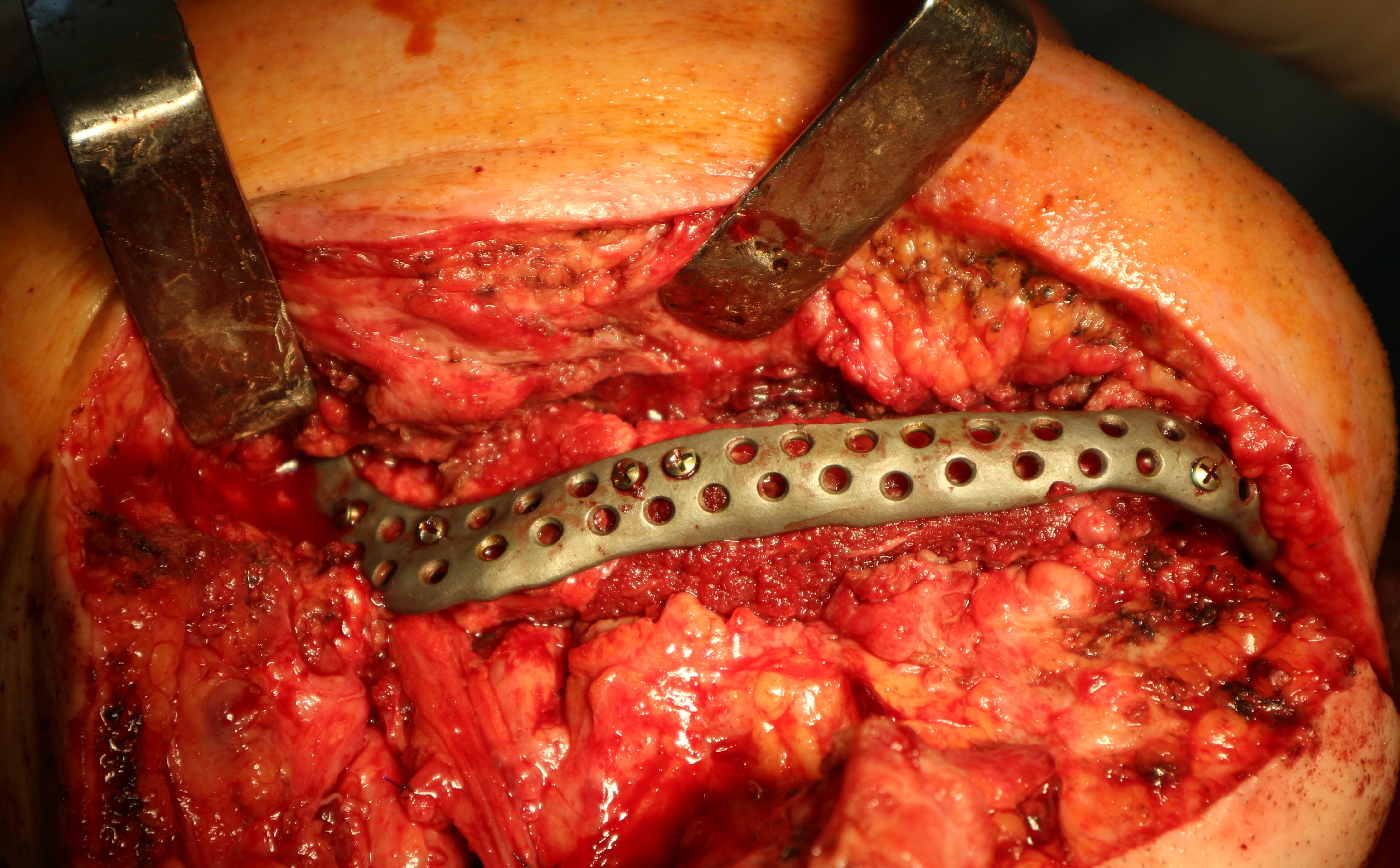
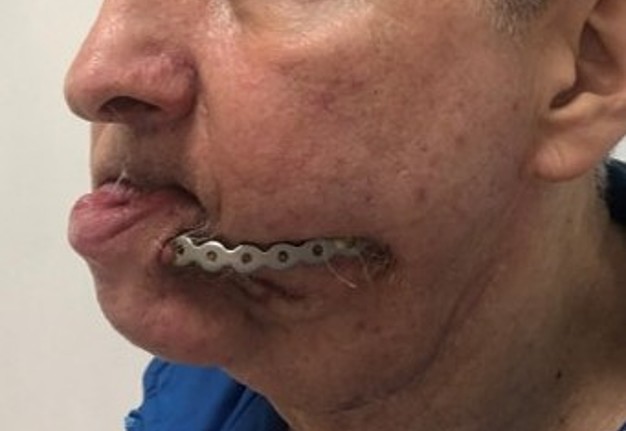
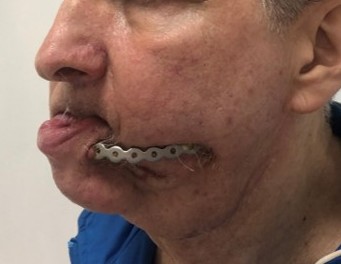
The main types of constructions used were anatomical titanium endoprostheses, which restored the lost areas of the mandible without additional use of bone autografts (Fig 4), patient-specific fixators in the form of a trough (Fig 5), and combined structures from elements of endoprosthesis and the patient-specific fixator (Fig 6).
Resection template made of polymeric bioinert sterilizable material used for accurate osteotomy presented at Figure 7 and the use of navigational surgical template for intraoperative adaptation of a bone autograft from the iliac crest – at Figure 8.
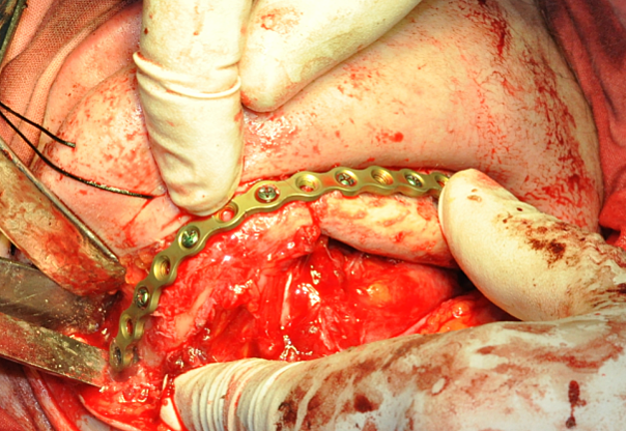
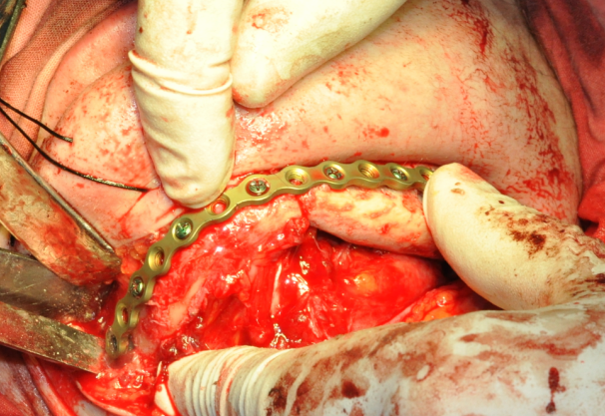
In the control group, after virtual repositioning of fragments and replacement of defects, plastic models of the mandible were made by stereolithography, which was used as a basis for bending of the reconstructive plates to give them the desired shape. Surgeries performed in accordance with the created virtual plan following the standards and protocols of reconstructive surgery of the jaws. 20
The clinical efficacy of the implemented approaches was judged based on local status assessment and postoperative computed tomography in 1-week, 3-, 6-, 12- and 24-month follow-up period.
The aesthetic result of surgery was analyzed on expert assessments using the following score scale: 5 points – changes are not visually noticeable, 4 points – changes in appearance are barely noticeable and do not affect the patient's quality of life, 3 points – there is an aesthetic deficit that does not require surgical correction, 2 points – there are aesthetic defects that require minor surgical corrections in the postoperative period, 1 point – there are significant aesthetic defects that require serious (often multi-stage surgical correction), and 0 points – the presence of severe aesthetic defects that cannot be eliminated.
Statistical calculations were performed in the software environment SPSS Statistics (version 18.0, SPSS, Chicago, IL, USA).
To determine the nature of the sample distribution, the Kolmogorov-Smirnov test for normality was used. Statistical analysis involved the calculation of mean values, standard deviation and mean error. The assessment of the reliability of discrepancies between the studied indicators was based on the use of nonparametric Mann-Whitney U test, parametric Student's t-test, and compliance criterion x2 (for qualitative and semiquantitative indicators).
Statistical discrepancies were considered significant at a confidence level of 95 percent (p < 0.05).
RESULTS
Defects localized within one site were noted in 9 (22.5 percent) patients of the main group and in 4 (20%) in the control group, within 2 anatomical areas – in 24 patients (60 percent) of the main group against 13 persons (65%) in the control group, within 3 and more anatomical sites – in 7 (17.5 percent) against 3 (15 percent) respectively.
Defects extended to the frontal part of the mandible in 11 patients (27.5 percent) of the main group and 6 patients (30%) in the control one, to the distal part of the body (on one or two sides) – in 29 patients (72.5 percent) of the main group and 19 patients (95%) of the control group, on the branch (on one or two sides) – in 32 patients (80%) of the main group and 14 patients (70%) of the control group.
The distribution of patients by the type of defect in clinical groups is shown in Table 1.
Surgical interventions for mandibular defects replacement performed in patients of 2 experimental groups were as follows: bone grafts were used in 17 patients of the main group (42.5 percent) and 13 of the control group (65%).
Among them, iliac crest grafts predominated (12 in the main [70.5 percent] [Fig 9] and 8 in control group [61.5 percent] [Fig 10]) of the fibula (4 in the main [22 percent] [Fig 11] and 3 in the control group [23 percent]).
Other types of bone grafts were bone blocks from intact areas of the mandible and metatarsophalangeal joints. In 23 observations of the main group (57.5 percent), the defects were completely replaced by anatomical endoprostheses of the mandible without bone transplantation.
In 7 patients (35 percent) of the control group, preformed reconstructive plates without bone grafting were used (this was considered mainly as a temporary solution before a full reconstruction, which was postponed for different reasons).
TABLE 1. Distribution of Patients of the Main and Control Groups by Defect Class According to Brown et al`s Classification (Fig 2). 21
| Defect Class | Main Group | Control Group |
| Class І, lateral defect without including canine | 29 ( 72.5%) | 11 (55%) |
| Class ІI, lateral defect include canine | 4 (10%) | 4 (20%) |
| Class ІІІ, anterior defect include two canines | 3 (7.5%) | 1 (5%) |
| Class ІV, bilateral defect include two canines | 4 (10%) | 4 (20%) |
FIGURE 11D. A 48-year-old male patient with ameloblastoma of the mandible in the right body and ramus. Stages of reconstruction with an individualized titanium implant and fibula transplant. D: installation and fixation of a patient-specific fixator with a bone autograft to the preserved fragment of the mandible.
The duration of surgery in the comparison groups did not differ significantly and averaged 231 ± 121 min in the main group against 233 ± 106 min in the control (р > 0.05).
After surgery, the anatomical shape of the mandible and its continuity were restored in all patients.
At the same time, in the main group, the aesthetic results were better: satisfactory results without the need for additional corrective surgeries were achieved in 57 percent of patients versus 10 percent in the control (p < 0.05).
The average score determined on the basis of expert assessment of the achieved aesthetic result in the main group was 2.88 + 1.5 against 1.75 + 1.2 in the control (p < 0.05).
The period of postoperative observation of patients ranged from 12 to 48 months and averaged 24 months in the main and 40 months in the control group.
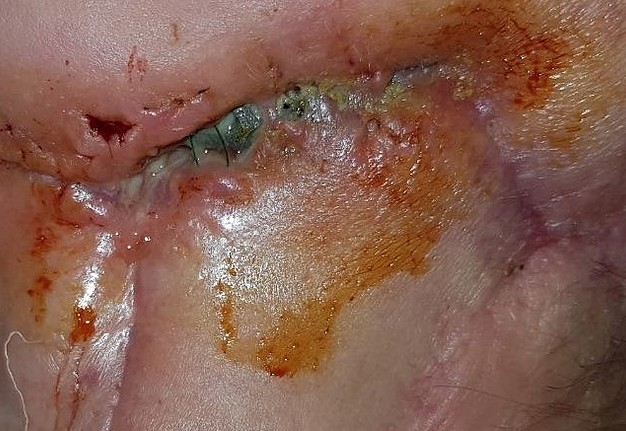
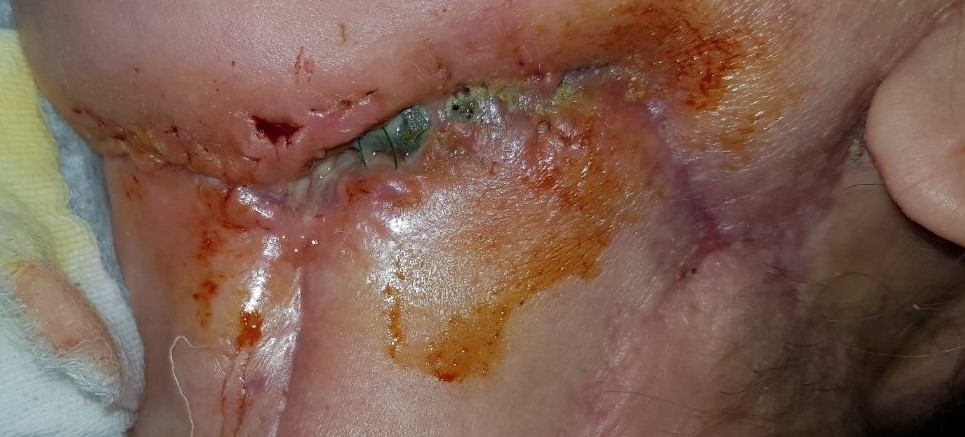
During this period, postoperative complications developed in 13 patients (32.5 percent) of the main group and 13 patients (65%) of the control group (р < 0.05) (Figs 12–15).
The list and distribution of complications in the study groups are given in the Table 2.
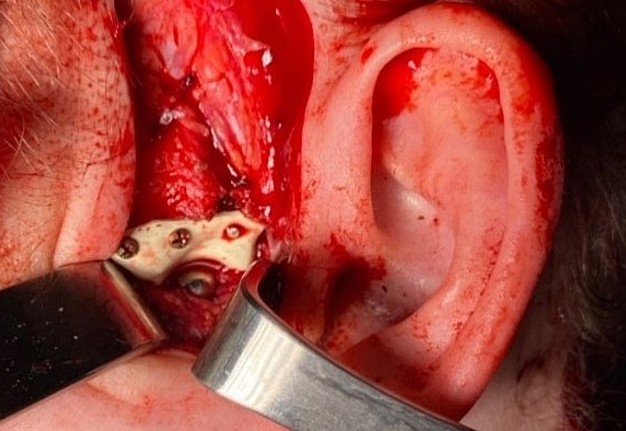
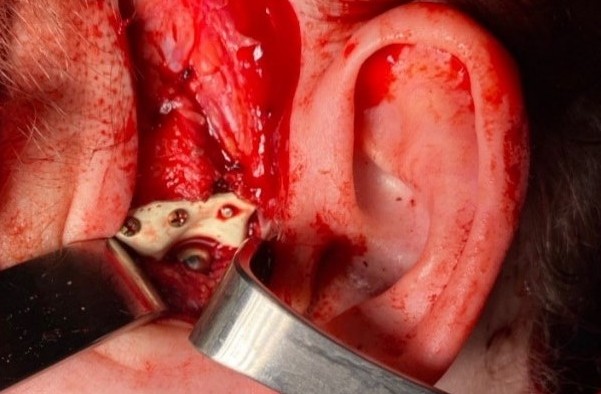
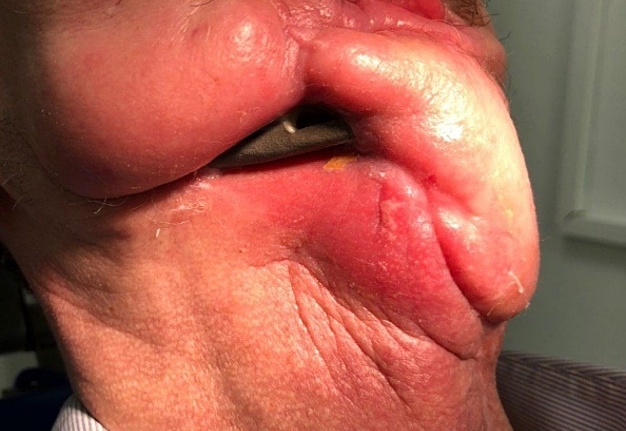
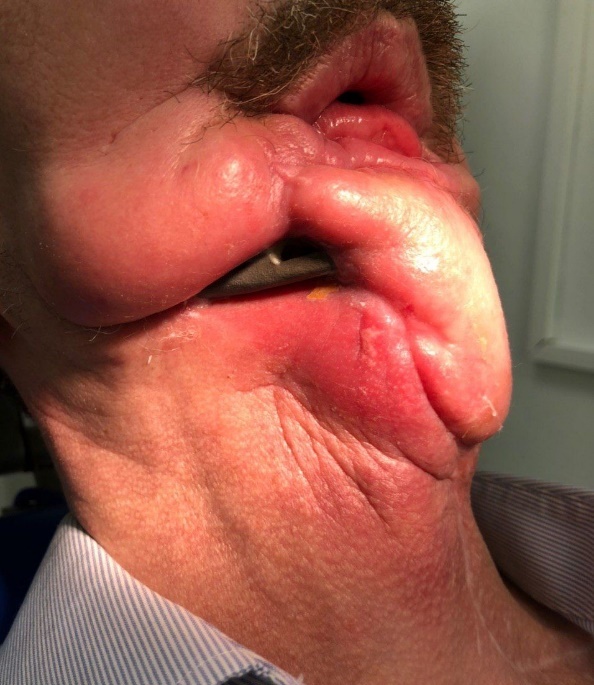
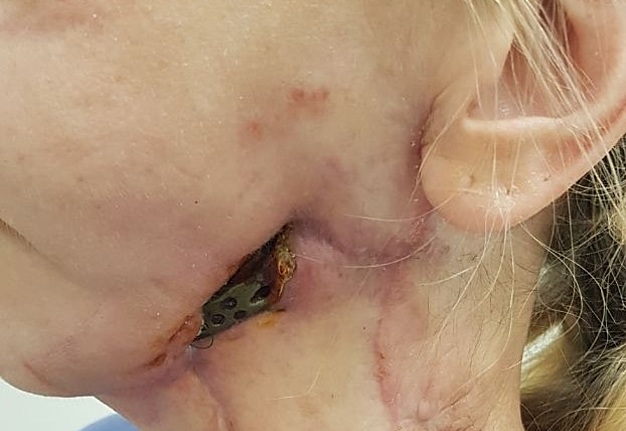
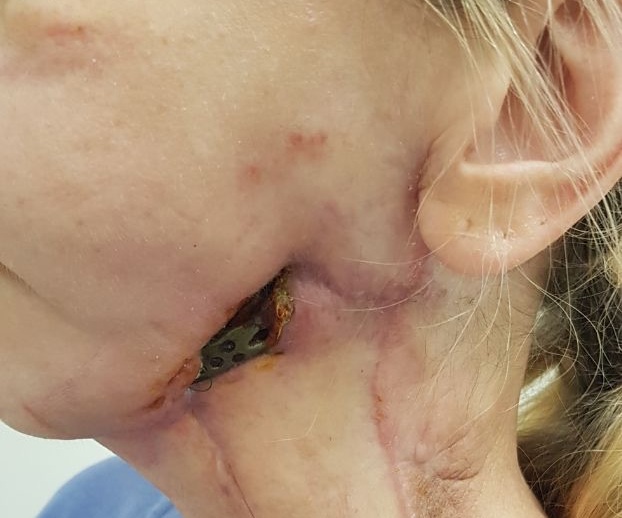
FIGURE 15A. Exposure of the individualized patient-specific endoprostheses. A: Exposure of patient-specific mandibular endoprosthesis in the anterior area and bilateral body after inflammatory complication and deterioration of soft tissue hemodynamics in the area of microvascular soft tissue autoplasty.
FIGURE 15B. Exposure of the individualized patient-specific endoprostheses. B: Exposure of a patient-specific mandibular endoprosthesis in the area of the anterior part, body and left ramus after an inflammatory complication and an attempt to close the soft tissue defect in the area of soft tissue autoplasty.
TABLE 2. Complications That Developed in Patients of the Main and Control Groups in the Postoperative Period.
| Main Group | Control Group | |
| The total number of patients with developed complications | 13 (32.5%)* | 13 (65%) |
| Complications of infectious and inflammatory nature | 9 (22.5%) | 8 (40%) |
| Partial or complete loss of the graft due to infection and sequestration | 6 (15%) | 4 (20%) |
| Implant/plate exposure | 8 (20%) | 7 (35%) |
| Removal of implants/fixators, with secondary reconstruction | 3 (7.5%) | 2 (10%) |
| Removal of implants/fixators, due to the tumor recurrence and the need for radiation and chemotherapy | 3 (7.5%) | 2 (10%) |
| Loosening and falling of the screws or plates` | 1 (2.5%)* | 8 (40%) |
| Secondary displacement / deformation of the fixator | 0* | 6 (30%) |
* – Differences with the control group are significant with р < 0.05.
Functional ability of the mandible in patients who underwent reconstructive surgery was restored during the year, after which the dynamics slowed down significantly and the functional state stabilized.
During this observation period, prosthetic structures in the area of bone grafting were manufactured in 7 patients (17.5 percent) of the main group and 5 patients (25%) in the control group (among them – fixed structures based on dental implants in 3 and 3 patients [7.5% and 15%], respectively).
Problems in occlusal relations that required orthopedic correction during this period persisted in 17.5 percent of patients in the main group and 25% of patients in the control group.
Pain and discomfort in the area of surgery associated with the movements of the mandible and chewing food were observed in 2.5 percent of patients in the main group and 25 percent of patients in the control group (p < 0.05). Most patients noted some limitations in mouth opening and mandibular movements.
Thus, in the main group, the maximum mouth opening was slightly limited (less than 1 cm) in 60% of patients, 1-2 cm in 37 percent, and more than 2 cm in 3%. In the control group, mouth opening was slightly limited (less than 1 cm) in 55% of patients, 1-2 cm in 40 percent, and more than 2 cm in 5 percent (discrepancies are insignificant, p > 0.05).
Lateral mandibular movements were sharply limited in 30 percent of patients in the main group and 25 percent of patients in the control group (p > 0.05).
Paresthesias in the area of innervation of the third branch of the trigeminal nerve were observed in 19 patients (47.5 percent) of the main group and 19 patients (95%) of the control group. Their occurrence was due to the mechanism of defect formation and its localization, and recovery was partial and very slow, regardless of the type of surgery used.
Despite certain functional limitations and residual aesthetic deficiency, 34 patients (85%) of the main group and 9 (45 percent) of the control group noted an improvement in their quality of life and
were satisfied with the results of the operation (р < 0.05).
DISCUSSION
Widespread introduction of computer simulation, modeling and CAD/CAM technologies in medicine, in particular in maxillofacial surgery, which has taken place over the past 2 decades, has qualitatively
changed approaches to the treatment of head and neck diseases and opened a new direction of facial skull reconstruction – computer associated surgery (ie., computer aided surgery) of the face.
It is based on algorithms that integrate modern capabilities of digital diagnostics, computer data processing, visualization, virtual simulation, three-dimensional design, intraoperative navigation, production of implants and medical devices. 22,23
Digital surgery involves the use of techniques and protocols that differ from traditional conceptually: it is based on the use of accurate calculations, computer simulation results and individualized medical devices, while classical methods of reconstruction of complex anatomical objects rely mainly on intraoperative manual approximation based and experience, practical skills and preferences of the surgeon, and not least depend on his intuition, spatial representation and creativity. 24
Our study contains a comparative analysis of the clinical effectiveness of PSI and traditional bone grafting techniques using preformed reconstructive plates in patients with mandibular defects. Short-
and long-term results of surgical interventions confirmed the presence of a number of advantages inherent in PSI, the main of which was to increase the accuracy of individual anatomical shape and
contour of the mandible, which probably affected the aesthetic outcome of surgery and patients` satisfaction level/quality of life.
It is known that the anatomy, architectonics, embryogenesis and conditions of the functional load of the mandible are unique and significantly different from other skeletal bones. This determines a significant discrepancy between the geometry of the mandible and bone grafts, regardless of the selected donor site.
Adaptation of the graft shape by performing osteotomies of mutual movement and additional mechanical processing of fragments does not allow effective restoring the features of the mandibular anatomy in areas with complex geometry (anterior part, angle, ramus, and condyle).
In these areas, PSIs have a significant advantage over traditional methods involving the use of preformed plates and bone blocks. They allow you to accurately restore the mandibular contour in the mirror image of the healthy side, compensating for the existing mismatch in the shape of the grafts.
Instead, when using traditional methods of defect replacement, there is often a need for contouring, correction of the mandibular shape, reproduction of the curvature of its contour using individualized
polymer and ceramic plates, bone grafts and more. This is fully confirmed by our data on the need for corrective surgery, which in the control group was twice as large. Aesthetic outcomes in the main group of patients, the satisfaction level and the assessment of changes in quality of life were probably better in patients with established PSI than in the control group.
Another advantage of PSIs identified in this work was the improvement of the biomechanical properties of the mandible-graft-fixator system and the probable reduction in the number of secondary displacements, loosening and prolapse of screws, fractures, etc. PSIs showed greater rigidity and strength compared to reconstructive plates, deformations or fractures of which occurred in 40 percent of cases. Thus, rigid reconstructive plates did not demonstrate the ability to withstand significant masticatory loads, especially in the presence of mandibular defects, loss of contact between bone fragments during their reconstruction and unfavorable cross-section of reparative regeneration.
The incidence of pain syndrome upon masticatory loads in the main group was also probably lower than in the control one, which confirms the ability of the placed patient-specific structures to perceive and redistribute the load effectively. According to a study, 25 increasing the rigidity and strength of installed titanium structures during their long-term function can cause a stress shielding effect, which negatively affects the processes of bone remodeling. This issue requires in-depth study and observation in the more distant postoperative period.
Despite the established benefits of PSIs, differences in the frequency of purulent-inflammatory complications, unsatisfactory clinical results with bone graft rejection, exposure and loss of titanium constructions in the comparison groups were insignificant. The use of PSI did not show significant benefits in restoring the functional state of the masticatory apparatus and the volume of mandibular
movements.
This, in our opinion, is due to the fact that the integral result of the operation is determined by many factors (topographic-anatomical, biomechanical, and biological), the general condition of the patient and the specific features of the clinical case. One of the main limitations of this study was that the structure of clinical groups on these parameters was quite heterogeneous, and the number of observations was too small to obtain statistically reliable results on certain parameters.
Thus, the current tendency to reduce the frequency of purulent-inflammatory complications with the use of PSIs (by 17.5 percent) in this number of observations was not significant. Restoration of mandibular function, occlusal-articulatory relationships, TMJ function, and muscular function were also independent of the type of performed surgery. The existing limitations were related to the difficulty of fixing and integrating the masticatory muscles to the titanium implant/endoprosthesis (especially the lateral pterygoid muscles, which limited the lateral mandibular movements in a significant percentage of cases), the inability to reproduce full range of motion in the TMJ (in modern constructions, usually, only rotational movements are restored) and the presence of significant soft tissues scarring in the area of defect.
With traditional approaches, this problem is even more pronounced, as fixation of muscles to bone grafts, ensuring their predictable restructuring and recovery of TMJ elements is extremely difficult to
achieve today. At the same time, modern methods of replacement of mandibular defects (in the main and control groups) allowed to achieve an acceptable level of compensation for lost functions and to
restore the ability to chew, swallow and speak in the vast majority of operated patients.
Thus, the use of PSI was associated with better aesthetic results and a lower frequency of complications compared to traditional methods of mandibular reconstruction.
However, their effect on function recovery of the damaged jaw and the frequency of purulent-inflammatory complications, as well as possible ways to reduce the frequency of unsatisfactory clinical results need further study.
CONCLUSIONS
The use of patient-specific implants made by the method of selective laser titanium sintering in patients with segmental and subtotal mandibular defects allows achieving satisfactory aesthetic and
functional results in 85% of operated patients. Postoperative complications in the form of purulent-inflammatory processes, exposure and graft/PSI rejection were noted in 22.5 percent of patients operated with this technology (against 40 percent in the control group); in 7.5 percent of patients (against 10% in the control) the development of these complications led to the removal of the established structure and the need for secondary mandibular reconstructions.
The use of PSIs compared to traditional methods of bone grafting, allows to achieve a more accurate restoration of the anatomical shape of the mandible in areas with complex geometry and probably
better aesthetic results, and significantly reduces the frequency of secondary displacement of bone fragments due to plastic deformation and destruction of fixation elements (p < 0.05).
At the same time, it probably does not affect the frequency of purulent-inflammatory complications, unsatisfactory clinical results and the effectiveness of the restoration of masticatory function in patients with mandibular defects.
TERM OF CONSENT
Written consent from patients was obtained to publish the clinical photographs.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ROLE OF THE AUTHORS
Denis M. Chernogorskyi (concept of the article,material collection, and writing), Marharyta V. Voller (material collection and writing), Aleksandr S. Vasilyev (material collection and writing), Yurii
V. Chepurnyi (material collection and editing), and Andrey V. Kopchak (concept of the article and editing). All authors read and approved the final manuscript.
FUNDINGS
No funding was received for this study.
ACKNOWLEDGMENTS
None.
REFERENCES (25)
-
Roser SM, Ramachandra S, Blair H, Grist W, Carlson GW, Christensen AM, Weimer KA, Steed MB. The accuracy of virtual surgical planning in free fibula mandibular reconstruction: comparison of planned and final results. J Oral Maxillofac Surg 2010;68(11):2824–32. Crossref | Medline | Google Scholar
-
Foley BD, Thayer WP, Honeybrook A, McKenna S, Press S. Mandibular reconstruction using computeraided design and computer-aided manufacturing: an analysis of surgical results. J Oral Maxillofac Surg 2013;71(2):e111–9. Crossref | Medline | Google Scholar
-
Bosc R, Hersant B, Carloni R, Niddam J, Bouhassira J, De Kermadec H, Bequignon E, Wojcik T, Julieron M, Meningaud, JP. Mandibular reconstruction after cancer: an in-house approach to manufacturing cutting guides. Int J Oral Maxillofac Surg 2017;46(1):24–31. Crossref | Medline | Google Scholar
-
Metzler P, Geiger EJ, Alcon A, Ma X, Steinbacher DM. Three-dimensional virtual surgery accuracy for free fibula mandibular reconstruction: planned versus actual results. J Oral Maxillofac Surg 2014;72(12):2601–12. Crossref | Medline | Google Scholar
-
Zheng L, Lv X, Zhang J, Liu S, Zhang J, Zhang Y. Translating computer-aided design and surgical planning into successful mandibular reconstruction using a vascularized iliac-crest flap. J Oral Maxillofac Surg 2017;76(4):886–93.Crossref | Medline | Google Scholar
-
Ciocca L, Mazzoni S, Marchetti C, Scotti R. Technical aspects of prosthetically guided maxillofacial surgery of the mandible. A pilot test study. Acta Bioeng Biomech 2014;16(2):21–9.
-
Mazzoni S, Marchetti C, Sgarzani R, Cipriani R, Scotti R, Ciocca L. Prosthetically guided maxillofacial surgery: evaluation of the accuracy of a surgical guide and custom-made bone plate in oncology patients after mandibular reconstruction. Plast Reconstr Surg 2013;131(6):1376–85. Crossref | Medline | Google Scholar
-
Stirling Craig E, Yuhasz M, Shah A, Blumberg J, Salomon J, Lowlicht R, Fusi S, Steinbacher DM. Simulated surgery and cutting guides enhance spatial positioning in free fibular mandibular reconstruction. Microsurg 2015;35(1):29–33. Crossref | Medline | Google Scholar
-
Antony AK, Chen WF, Kolokythas A, Weimer KA, Cohen MN. Use of virtual surgery and stereolithography-guided osteotomy for mandibular reconstruction with the free fibula. Plast Reconstr Surg 2011;128(5):1080–4. Crossref | Medline | Google Scholar
-
Huang JW, Shan XF, Lu XG, Cai ZG. Preliminary clinic study on computer-assisted mandibular reconstruction: the positive role of surgical navigation technique. Maxillofac Plast Reconstr Surg 2015;37(1):20.
-
Mascha F, Winter K, Pietzka S, Heufelder M, Schramm A, Wilde F. Accuracy of computer-assisted mandibular reconstructions using patient-specific implants in combination with CAD/CAM
fabricated transfer keys. J Craniomaxillofac Surg 2017;45(11):1884–97. -
Mascha F, Winter K, Pietzka S, Heufelder M, Schramm A, Wilde F. Accuracy of computer-assisted mandibular reconstructions using patient-specific implants in combination with CAD/CAM fabricated transfer keys. J Craniomaxillofac Surg 2017;45(11):1884–97. Crossref | Medline | Google Scholar
-
Schepers RH, Raghoebar GM, Vissink A, Stenekes MW, Kraeima J, Roodenburg JL, Reintsema H, Witjes MJ. Accuracy of fibula reconstruction using patient-specific CAD/CAM reconstruction plates and dental implants: a new modality for functional reconstruction of mandibular defects. J Craniomaxillofac Surg 2015;43(5):649–57. Crossref | Medline | Google Scholar
-
Schepers RH, Raghoebar GM, Vissink A, Stenekes MW, Kraeima J, Roodenburg JL, Reintsema H, Witjes MJ. Accuracy of fibula reconstruction using patient-specific CAD/CAM reconstruction plates and dental implants: a new modality for functional reconstruction of mandibular defects. J Craniomaxillofac Surg 2015;43(5):649–57. Crossref | Medline | Google Scholar
-
Leiggener C, Messo E, Thor A, Zeilhofer HF, J-M Hirsch. A selective laser sintering guide for transferring a virtual plan to real time surgery in composite
mandibular reconstruction with free fibula osseous flaps. Int J Oral Maxillofac Surg 2009;38(2):187–92. Crossref | Medline | Google Scholar -
Seruya M, Fisher M, Rodriguez ED. Computer-assisted versus conventional free fibula flap technique for craniofacial reconstruction: an outcomes comparison. Plast Reconstr Surg 2013;132(5):1219–28. Crossref | Medline | Google Scholar
-
Peel S, Bhatia S, Eggbeer D, Morris DS, Hayhurst C. Evolution of design considerations in complex craniofacial reconstruction using patient-specific implants. Proc Inst Mech Eng H 2017;231(6):509–24. Crossref | Medline | Google Scholar
-
Sang-Hoon Kang, Moon-Key Kim, Ji-Yeon Lee. Single-tooth dento-osseous osteotomy with a computer-aided design/computer-aided manufacturing surgical guide. J Korean Assoc Oral Maxillofac Surg 2016;42(2):127–30. Crossref | Medline | Google Scholar
-
Zhang L, Liu Z, Li B, Yu H, Shen SG, Wang X. Evaluation of computer-assisted mandibular reconstruction with vascularized fibular flap compared to conventional surgery. Oral Surg Oral
Med Oral Pathol Oral Radiol 2016;121(2):139–48. Crossref | Medline | Google Scholar -
Boffano P, Corre P, Righi S. The role of intra-articular surgery in the management of mandibular condylar head fractures. Atlas Oral Maxillofac Surg Clin North Am 2017;25(1):25–34. Crossref | Medline | Google Scholar
-
Brown JS, Barry C, Ho M, Shaw R. A new classification for mandibular defects after oncological resection. Lancet Oncol 2016;17(1):e23–e30. Crossref | Medline | Google Scholar
-
Ciocca L, Mazzoni S, Fantini M, Persiani F, Marchetti C, Scotti R. CAD/CAM guided secondary mandibular reconstruction of a discontinuity defect after ablative cancer surgery. J Craniomaxillofac Surg 2012;40(8):e511–5. Crossref | Medline | Google Scholar
-
Ciocca L, Mazzoni S, Marchetti C, Scotti R. Technical aspects of prosthetically guided maxillofacial surgery of the mandible. A pilot test study. Acta Bioeng Biomech 2014;16(2):21–9.
-
Roser SM, Ramachandra S, Blair H, Grist W, Carlson GW, Christensen AM, Weimer KA, Steed MB. The accuracy of virtual surgical planning in free fibula mandibular reconstruction: comparison of planned and final results. J Oral Maxillofac Surg 2010;68(11):2824–32.Crossref | Medline | Google Scholar
-
Shayesteh Moghaddam N. Toward patient specific long lasting metallic implants for mandibular segmental defects. Toledo, OH, United States: The University of Toledo; 2015.