Ultrasonographic Identification of the Parotid Cystadenolymphoma (Warthin’s Tumor) by Oral and Maxillofacial Surgeons: Supplement to the Matsuda and Colleagues’ Classification
July 31, 2022
https://doi.org/10.23999/j.dtomp.2022.7.1
J Diagn Treat Oral Maxillofac Pathol 2022;6: 92–110.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE
Cherniak OS, Savchuk LA, Ripolovska OV, Demidov VH, Nozhenko OA, Zaritska VI, Snisarevskyi PP. Ultrasonographic identification of the parotid cystadenolymphoma (Warthin’s tumor) by oral and maxillofacial surgeons: supplement to the Matsuda and colleagues’ classification. J Diagn Treat Oral Maxillofac Pathol 2022;6(7):92–110.
NATIONAL REPOSITORY OF ACADEMIC TEXTS
https://nrat.ukrintei.ua/en/searchdoc/2022U000191/
SUMMARY
Warthin’s tumor (WT), which is also known as papillary cystadenoma lymphomatosum, monomorphic parotid adenoma, adenolymphoma, cystadenolymphoma, and branchiogenic adenoma, is to be differentiated (in surgical practice) from other parotid masses. The purposes of our retrospective case series study are: (1) to describe ultrasound morphology (sonomorphology) of the WT in patients referred to our hospital, (2) based on the presented cases to propose a supplement to the Matsuda and colleagues’ classification (2017) of anechoic area patterns of the WT, and (3) to expand the knowledge of oral and maxillofacial surgeons for the preoperative ultrasonographic verification of the WT and for choosing the most appropriate surgical technique. Over three years, 5 patients (mean age, 65.4 years) with parotid WT had been examined with gray-scale, color, and power Doppler ultrasonography. Cystic components are visualized in all five WT cases but in different proportions. Case 1 and 4 showed the presence of septations. According to Matsuda and colleagues’ (2017) classification of anechoic area patterns, in our cases the US patterns of the WTs belong only to Group 3 (i.e., with large anechoic areas) (n = 4) and Group 4 (multiple and sponge-like anechoic areas) (n = 1). Moreover, based on the presented five cases, we offer an addition to the classification of Japanese authors. In conclusion, our supplement to Matsuda and colleagues’ classification of anechoic area patterns of the WT can help surgeons around the globe to be more accurate in preoperative verification of cystadenolymphoma. This case series illustrate the growing importance of ultrasonography in the professional life of oral and maxillofacial and head and neck surgeons. Based on the cystic structure of this benign tumor and the ultrasound appearance presented in our case series, we propose to continue using the term “cystadenolymphoma” with a purpose to emphasize the tumor`s structure.
INTRODUCTION
In 1929, Aldred Scott Warthin was the next one, after Hilderand (1895), and Albrecht and Arzt (1910), who described two similar lower pole–located parotid tumors and named such neoplasm as papillary cystadenoma lymphomatosum.1–4 There are other names appeared from 1929 which describe this pathological entity.4 Among them monomorphic parotid adenoma,5 adenolymphoma, cystadenolymphoma,6,7 branchiogenic adenoma,8 and Warthin’s tumor (WT)9,10. The chronology of WT naming is perfectly depicted in the Jerry Chapnik’s study (1983).4 WT is a second (29.2 percent) most popular parotid benign tumor after the pleomorphic adenoma (65.6 percent) (Pinkston and Cole, 1999).11 Despite WT is predominantly founded in parotid glands, some authors report cases of nasopharyngeal adenolymphoma12, laryngeal adenolymphoma13,14 or even arising from minor salivary glands15.
Literature revealed that WTs can have parotid and extraparotid location.10 Snyderman et al (1986) also noted that adenolymphomas can arise from the periparotid lymph nodes.16 According to Hellquist and Skalova (2014), parotid WT is usually noted in the caudal pole of the parotid gland―the part of the gland that has the most intraparotid lymph nodes.10
Unfortunately, malignization of WT is possible. That was proved by Yamada et al (2002) and Yu et al (2016) who described the cases of mucoepidermoid carcinoma arising in Warthin’s tumor.17,18
Choosing the best imaging for establishing the correct preoperative diagnosis is crucial. Kim et al (2004) emphasized that such preoperative imaging as ultrasonography (US) can help reaching two goals in parotid region―(1) determination of the probable histologic diagnosis of the parotid mass and (2) determination of the extent of the operation.19
Usually, the differential diagnosis in case of parotid mass with no facial nerve dysfunction should be made between mixed (i.e., pleomorphic adenoma) and WT. Many studies describe the US appearance of the WT.19–25 But, the work of Matsuda and colleagues’ (2017) is especially worth of attention due to the novel US classification which can be helpful both to sonographers and to oral and maxillofacial surgeons.25
The purposes of our retrospective case series study are: (1) to describe ultrasound morphology (sonomorphology) of the WT in patients referred to our hospital, (2) based on the presented cases to propose a supplement to the Matsuda and colleagues’ classification (2017) of anechoic area patterns of the WT,25 and (3) to expand the knowledge of oral and maxillofacial surgeons for the preoperative ultrasonographic verification of the WT and for choosing the most appropriate surgical technique.
MATERIALS AND METHODS
We retrospectively reviewed the clinical, ultrasonographic, intraoperative, macroscopic, and histopathologic data of patients with parotid adenolymphoma (i.e., WT) at Kyiv Regional Clinical Hospital between December 2014 and June 2016. US and its interpretation have been done by three experienced doctors of ultrasound diagnostics (O.S.C., her experience is 16 years; O.V.R., her experience is 29 years; L.A.S., her experience is 31 years). US was performed on two ultrasound machines (HD11 XE, the Philips, Amsterdam, the Netherlands) using linear array probe (also known as linear transducer) (the Philips L12-3 [12-3 MHz], Philips, Amsterdam, the Netherlands) and curved array probes (also known as convex transducer) (Philips C5-2 [5-2 MHz], Amsterdam, the Netherlands). The sample included five patients (3 male and 2 female patients) with a mean age of 65.4 years (range, 49 to 78 years). Patients underwent parotid tumors removal applying extracapsular dissection with facial nerve dissection (Gleave, 1995; Witt and Rassekh, 2018) and partial parotidectomy.26,27 All five patients signed an informed consent agreement. We evaluated 5 tumors that were histopathologically diagnosed as Warthin’s tumor between December 2014 and June 2016. Histological diagnosis of adenolymphoma in all five cases was verified by two experienced pathologists (V.I.Z., her experience is 23 years; P.P.S., his experience is 18 years). Gray-scale ultrasound images (also known as ‘B-mode ultrasound images’) were used for the tumors’ long-to-short diameter measurements.
For the assessment of ultrasound patterns of WTs we used Matsuda and colleagues’ (2017) classification of anechoic area patterns and their criteria for the assessment of ultrasonographic features of the parotid tumors.25 We obtained approval for this retrospective review of patients’ data from the Kyiv Regional Clinical Hospital review board.
RESULTS
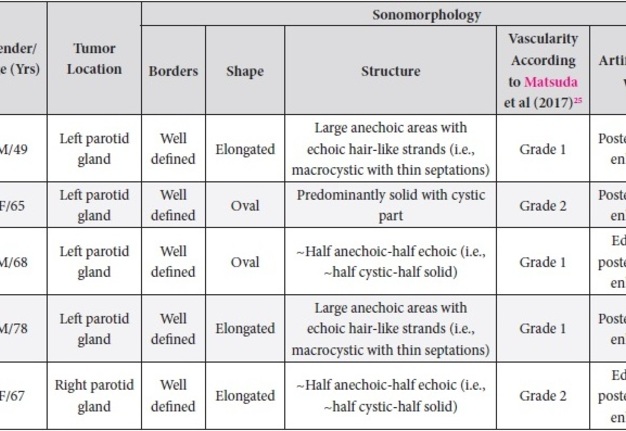
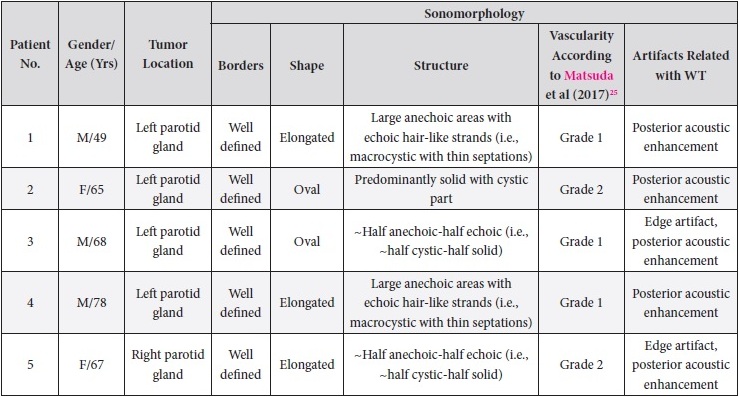
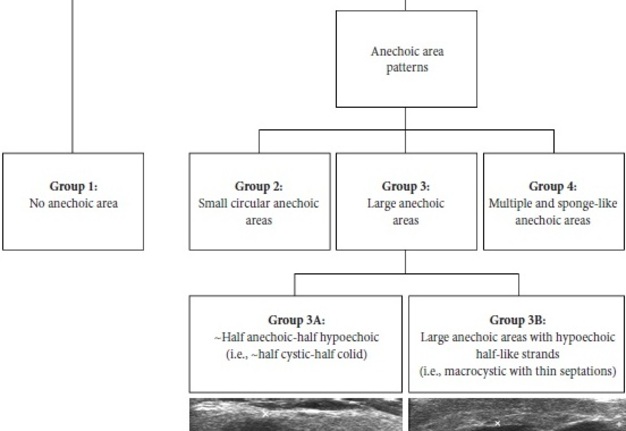
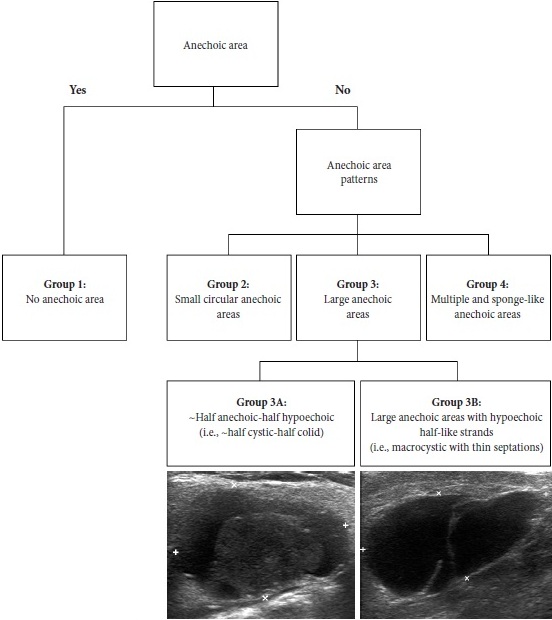
Results of our case series study are presented in Tables 1 and 2. Male to female ration in our retrospective case study was 3:2. The average age of the managed patients was 65.4 years. Cystic components are visualized in all five WT cases but in different proportions. Case 1 and 4 showed the presence of septations. According to Matsuda and colleagues’ (2017) classification of anechoic area patterns,25 in our cases the US patterns of the WTs belong only to Group 3 (i.e., with large anechoic areas) (n = 4) and Group 4 (multiple and sponge-like anechoic areas) (n = 1). Moreover, based on the presented five cases, we offer an addition to the classification of Japanese authors. The Group 3 can be divided into Group A and B depending on the cystic chambers volume. Group 3A will include Warthin’s tumors that show “~half anechoic-half echoic” ultrasound appearance (i.e., ~half cystic-half solid structure) (what is confirmed by Case 1, 3, and 5 in our study) and the Group 3B will include large anechoic areas with echoic hair-like strands (i.e., macrocystic ultrasound appearance with thin septations) (confirmed by Case 4). Edge artifact and posterior acoustic enhancement artifact were two common US artifacts noted on the exanimated sonograms. A mean long-to-short diameter of WTs was 3.14 × 2.01 cm (range of long diameter, 2.31 to 4.0 cm; range of short diameter, 1.37 to 3.1 cm).
TABLE 1. Sonomorphology of the Five Parotid Adenolymphomas Managed in Our Hospital.
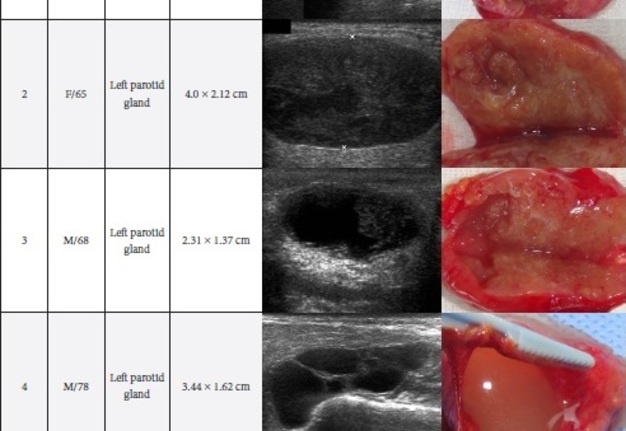
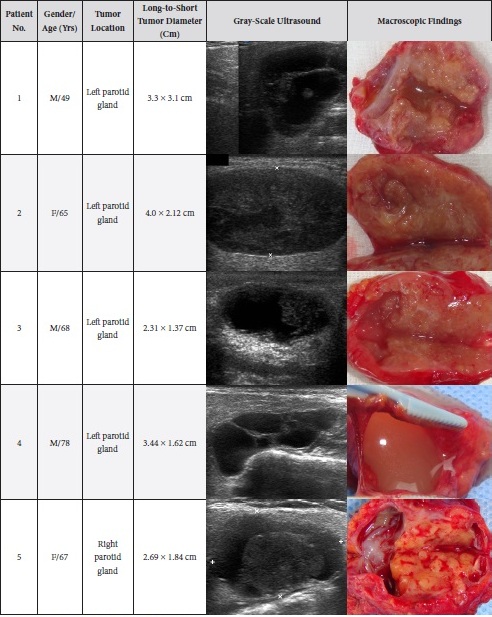
TABLE 2. Comparison of Sonomorphology and Gross View of the Removed Adenolymphomas.
CASE SERIES
~HALF CYSTIC-HALF SOLID: MATSUDA ET AL GROUP 3A (CASE 1)
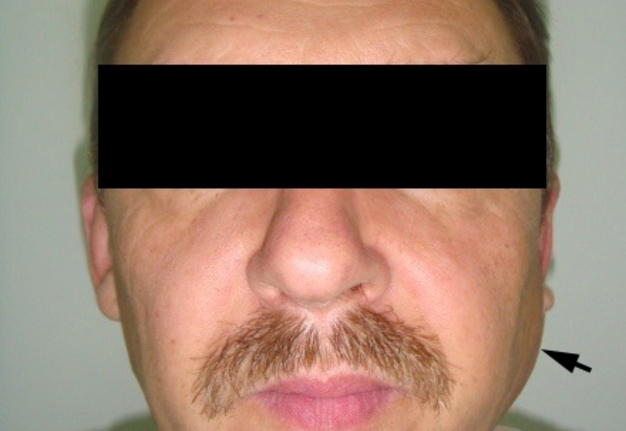
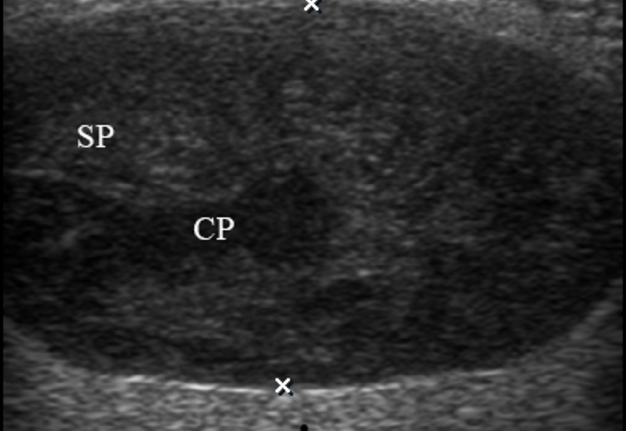
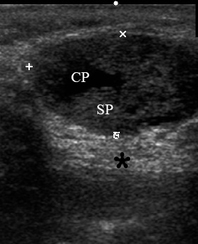
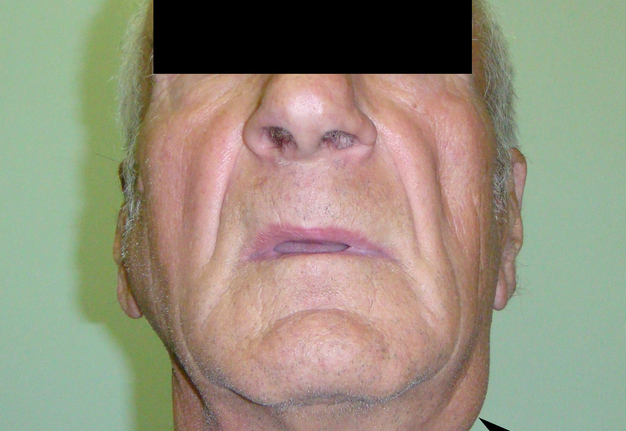
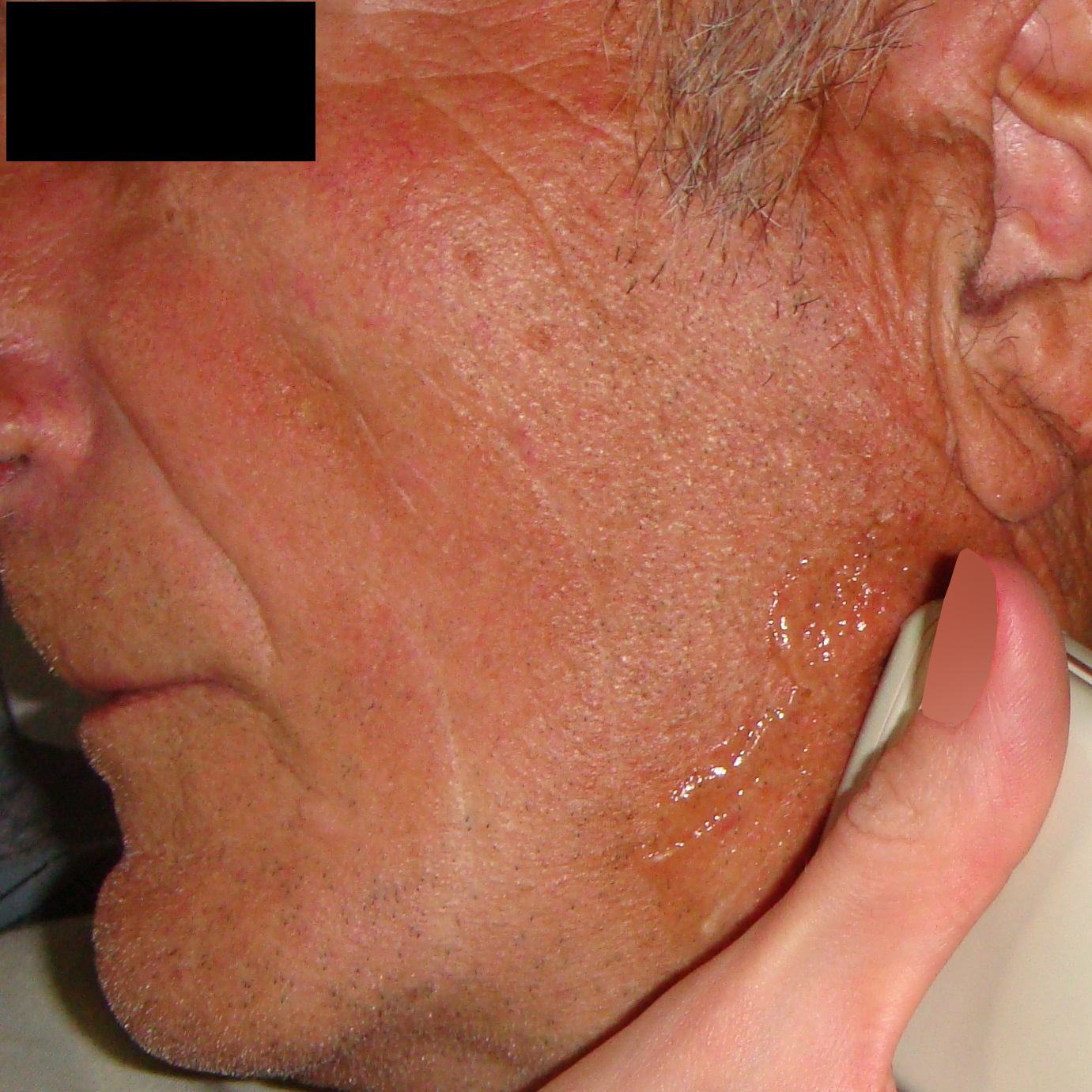
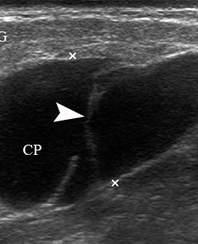
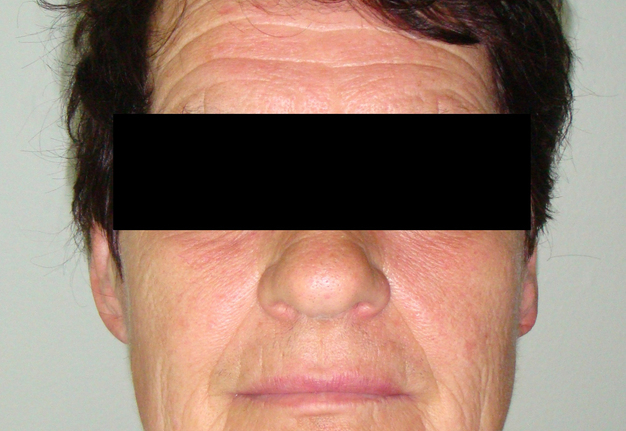
A 49-year-old male was referred to the Kyiv Regional Clinical Hospital in December 2014 with a facial asymmetry in the left parotid region (Fig 1). A palpable painless movable lesion in the projection of left parotid gland was noted. Patient reported slow growth of the lesion during last years. Gray-scale US revealed parotid mass of the left parotid gland with multicystic structure and septations (Fig 2). The long-to-short tumor diameter reached 3.3 × 3.1 cm. US appearance of the mass corresponds to the Group 3 of Matsuda et al classification (2017)25 of anechoic area patterns and Grade 1 vascularization (two flow signals were noted). The surgery (tumor removal with extracapsular dissection and facial nerve dissection) was done by V.H.D. under general anesthesia and the diagnosis of adenolymphoma was established by pathologists (V.I.Z. and P.P.S.).
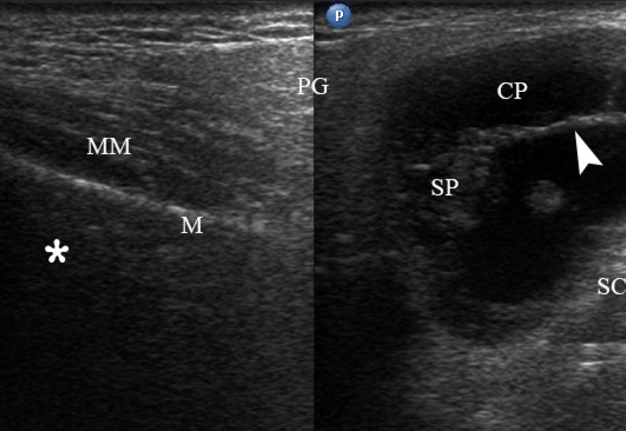
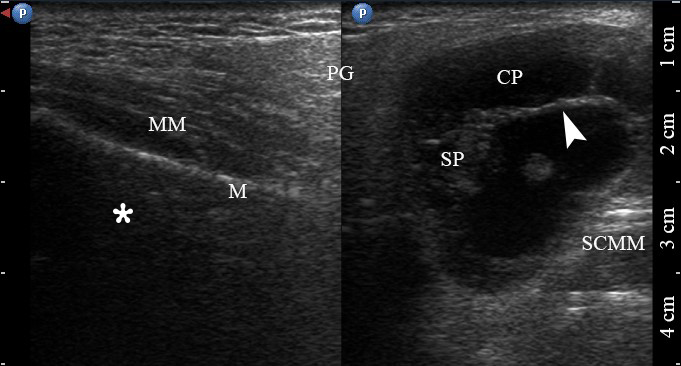
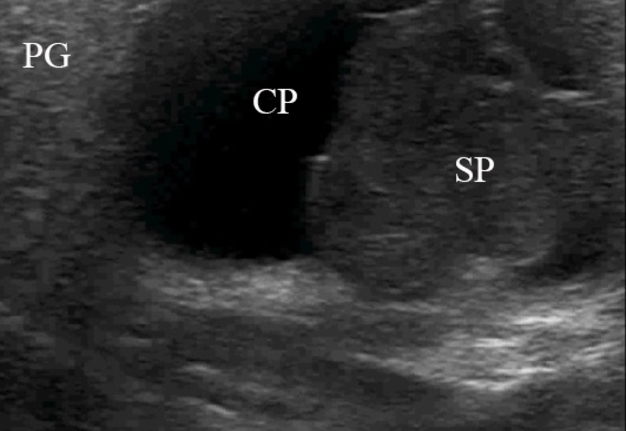
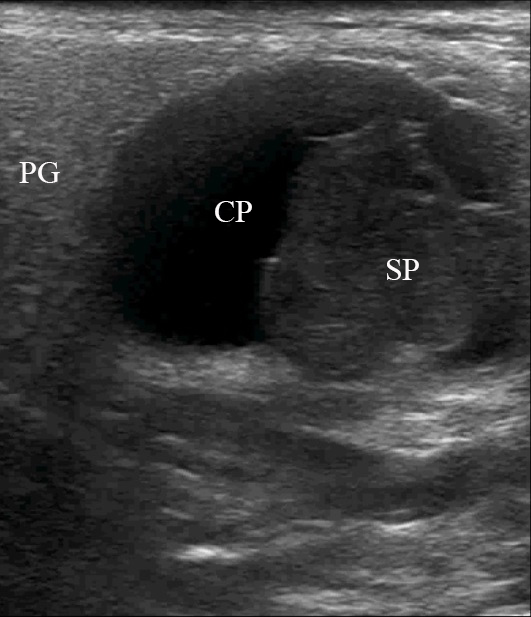
FIGURE 2. Case 1. A 49-year-old male patient. Conjoined gray-scale sonograms of WT of the left parotid gland. Notes tumor’s multicystic structure with septations (arrowhead). Tumor’s borders are well defined, echogenicity is mixed hypoechoic. PG, parotid gland, CP, cystic part of the tumor; SP, solid part of the tumor; M, mandible; MM, masseter muscle; SCMM, sternocleidomastoid muscle. Asterisk labels posterior acoustic shadowing deeper to the superficial surface of mandible. The depth of sonograms is 4.0 cm. Letter “P” inside the blue circle at the upper left corners of the sonograms indicates on the probe’s side (corresponds to the probe bump on the left side). Printed with permission and copyrights retained by O.S.C.
MULTIPLE AND SPONGE-LIKE ANECHOIC AREAS: MATSUDA ET AL GROUP 4 (CASE 2)
A 65-year-old Caucasian female was referred to the Kyiv Regional Clinical Hospital in June 2015 due to the facial asymmetry in the region of left parotid gland. US (Fig 3) showed an intraparotid localization of oval tumor with well-defined borders, mixed hypoechoic structure which measured 4.0 × 2.12 cm. US appearance of the mass corresponds to the Group 4 (multiple and sponge-like anechoic areas) of Matsuda et al classification (2017)25 of anechoic area patterns and Grade 2 vascularization (several linear flow signals). Tumor removal (with extracapsular dissection and facial nerve dissection) was done by V.H.D. under general anesthesia. The histological examination (P.P.S. and V.I.Z.) confirmed the “adenolymphoma” diagnosis suspected by preoperative ultrasound imaging.
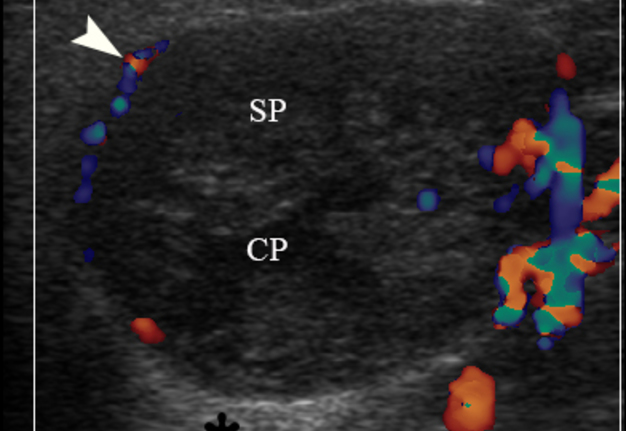
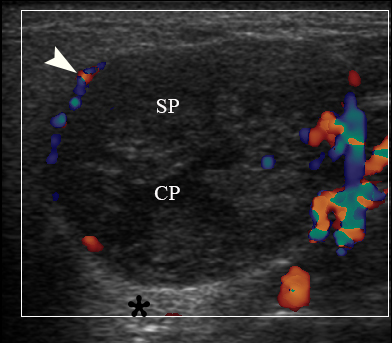
FIGURE 3A. Case 2. Preoperative gray-scale (A) and color Doppler (C) sonograms in a 65-year-old female with a WT of the left parotid gland. Sonograms show an oval tumor with well-defined borders, mixed hypoechoic structure which measured 4.0 × 2.12 cm. Two ‘×’ calipers at the image A measure the tumor’s transverse size (2.12 cm). Notes multiple and sponge-like anechoic areas. CP, cystic part (anechoic area) of the mass; SP, solid part mass. The artifact of acoustic enhancement is labeled by asterisk. Color Doppler US revealed vascularization of the tumor (arrowhead). Images B and D show positions of the linear transducer. The depth of sonograms is 3.0 cm. Printed with permission and copyrights retained by O.S.C.
FIGURE 3B. Case 2. Preoperative gray-scale (A) and color Doppler (C) sonograms in a 65-year-old female with a WT of the left parotid gland. Sonograms show an oval tumor with well-defined borders, mixed hypoechoic structure which measured 4.0 × 2.12 cm. Two ‘×’ calipers at the image A measure the tumor’s transverse size (2.12 cm). Notes multiple and sponge-like anechoic areas. CP, cystic part (anechoic area) of the mass; SP, solid part mass. The artifact of acoustic enhancement is labeled by asterisk. Color Doppler US revealed vascularization of the tumor (arrowhead). Images B and D show positions of the linear transducer. The depth of sonograms is 3.0 cm. Printed with permission and copyrights retained by O.S.C.
FIGURE 3C. Case 2. Preoperative gray-scale (A) and color Doppler (C) sonograms in a 65-year-old female with a WT of the left parotid gland. Sonograms show an oval tumor with well-defined borders, mixed hypoechoic structure which measured 4.0 × 2.12 cm. Two ‘×’ calipers at the image A measure the tumor’s transverse size (2.12 cm). Notes multiple and sponge-like anechoic areas. CP, cystic part (anechoic area) of the mass; SP, solid part mass. The artifact of acoustic enhancement is labeled by asterisk. Color Doppler US revealed vascularization of the tumor (arrowhead). Images B and D show positions of the linear transducer. The depth of sonograms is 3.0 cm. Printed with permission and copyrights retained by O.S.C.
FIGURE 3D. Case 2. Preoperative gray-scale (A) and color Doppler (C) sonograms in a 65-year-old female with a WT of the left parotid gland. Sonograms show an oval tumor with well-defined borders, mixed hypoechoic structure which measured 4.0 × 2.12 cm. Two ‘×’ calipers at the image A measure the tumor’s transverse size (2.12 cm). Notes multiple and sponge-like anechoic areas. CP, cystic part (anechoic area) of the mass; SP, solid part mass. The artifact of acoustic enhancement is labeled by asterisk. Color Doppler US revealed vascularization of the tumor (arrowhead). Images B and D show positions of the linear transducer. The depth of sonograms is 3.0 cm. Printed with permission and copyrights retained by O.S.C.
~HALF CYSTIC-HALF SOLID: MATSUDA ET AL GROUP 3A (CASE 3)
A 68-year-old Caucasian male was referred to the Kyiv Regional Clinical Hospital in September 2015 with a palpable mass in a left retromandibular region. Gray-scale US (Fig 4) showed parotid localization of oval tumor with well-defined borders, mixed hypoechoic structure. The long-to-short diameter of the WT reached 2.31 × 1.37 cm. US appearance of the mass corresponds to the Group 3 of Matsuda et al classification (2017)25 of anechoic area patterns and Grade 1 vascularization (with no flow signal). Tumor removal (including extracapsular dissection) was done by V.H.D. under the general anesthesia. Adenolymphoma was established by pathologists (P.P.S. and V.I.Z.) as a histological diagnosis.
FIGURE 4. Case 3. A 68-year-old male patient. Preoperative gray-scale transversal (A) and longitudinal (C) sonograms of WT in the left parotid gland. CP, cystic part of WT; SP, solid part of the WT. Asterisk labels the artifact of acoustic enhancement and two circles indicate the edge artifact. Images B and D show positions of the linear probe. The depth of this cropped sonogram is 3.0 cm. Calipers ‘+’ and ‘×’ measure the long-to-short WT diameter (A) which reaches 1.88 × 1.28 cm. The tumor lies at a depth of 1.67 cm (distance between medial border of the tumor and skin surface). Longitudinal size of the WT (distance between calipers ‘×’ and ‘+’) reach 2.31 × 1.37 cm. The tumor lies at a depth of 1.65 cm (distance between medial border of the tumor and skin surface). Artifact of the posterior acoustic enhancement is stronger in those posterior areas that were located behind the areas of the tumor with a greater number or size of cystic cavities. The edge artifact (visualized as acoustic shadowing artifact) is labeled by two circles. PG, parotid gland; AAS, artifact of acoustic shadowing (typical for fluid content). Printed with permission and copyrights retained by O.V.R.
MACROCYSTIC WITH SEPTATIONS: MATSUDA ET AL GROUP 3B (CASE 4)
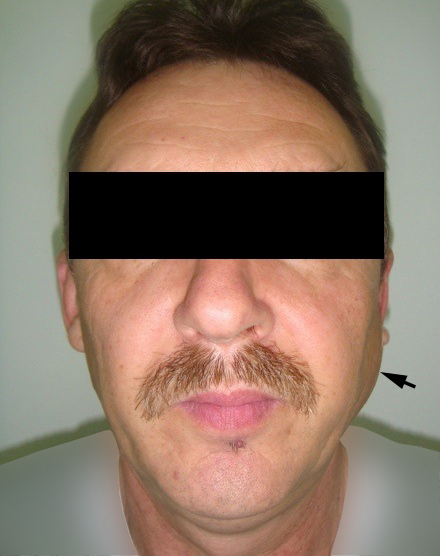
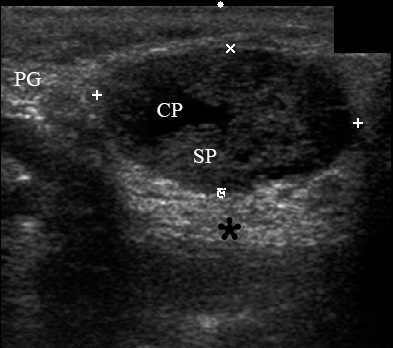
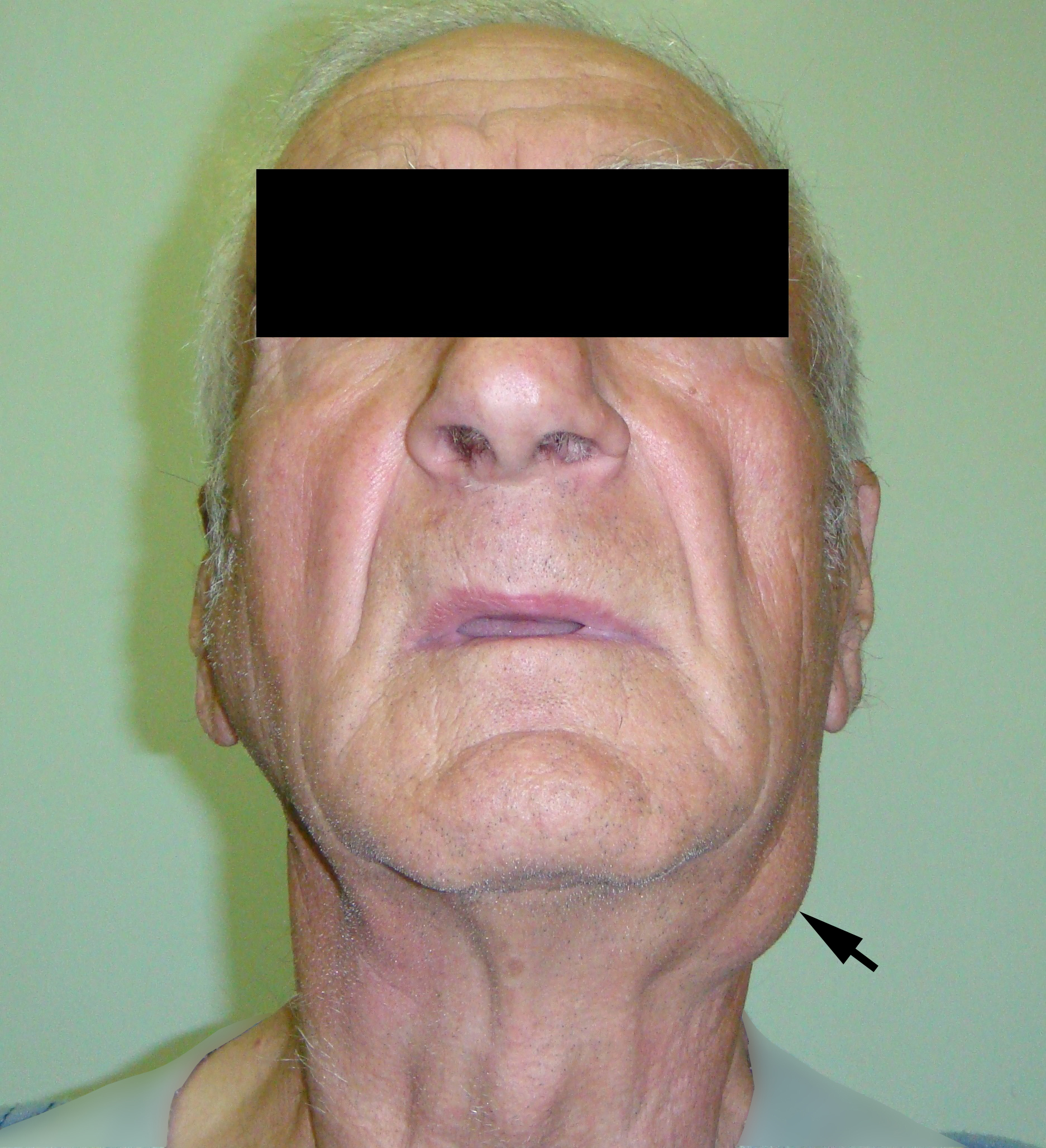
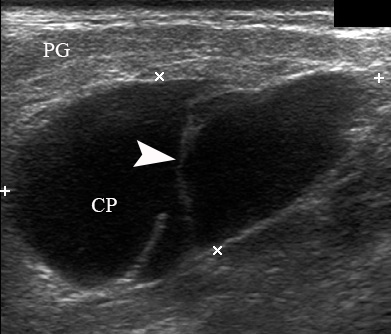
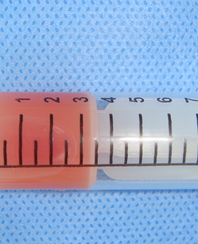
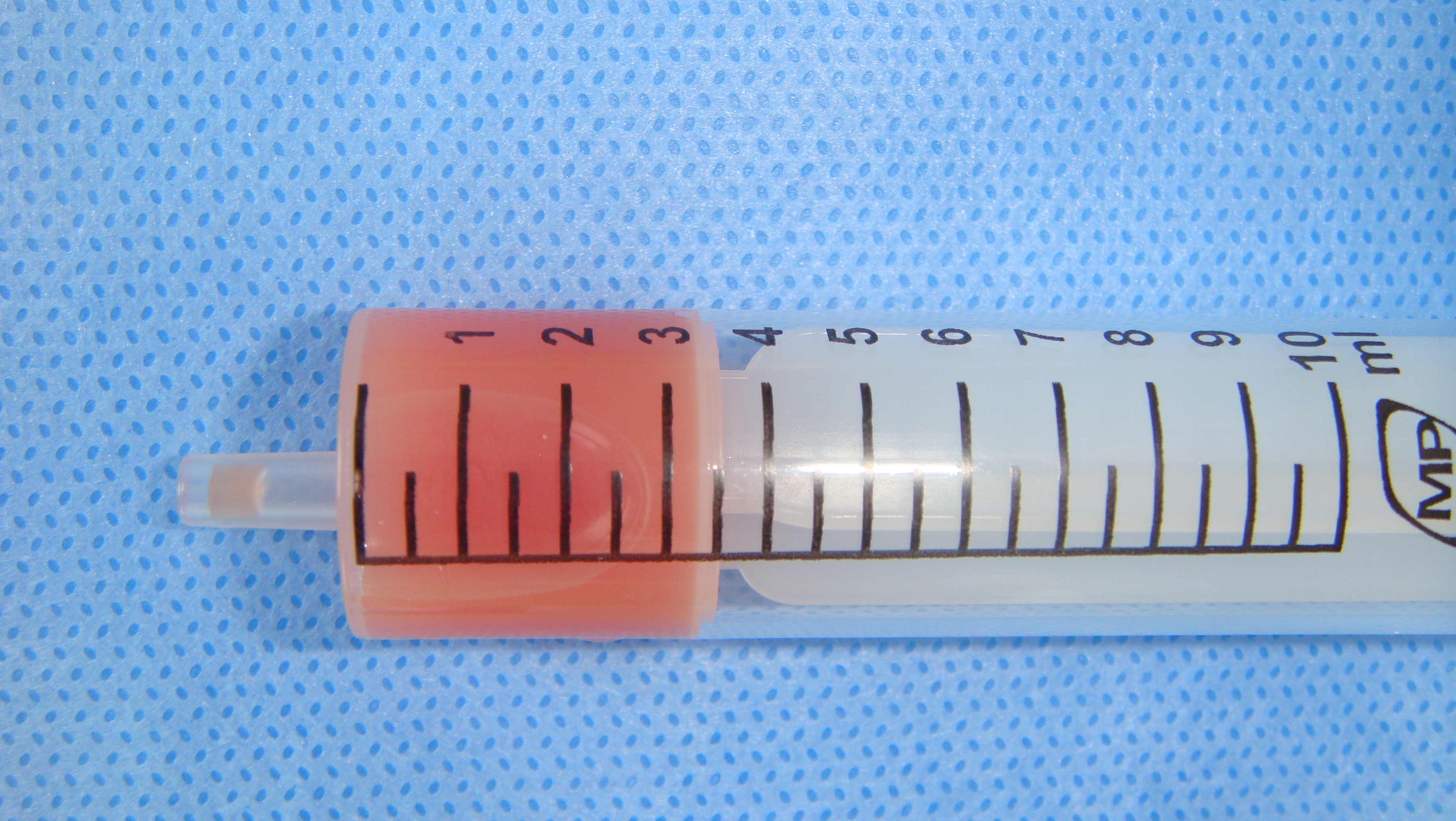
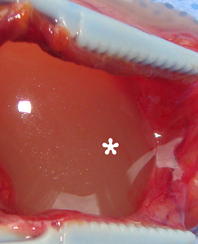
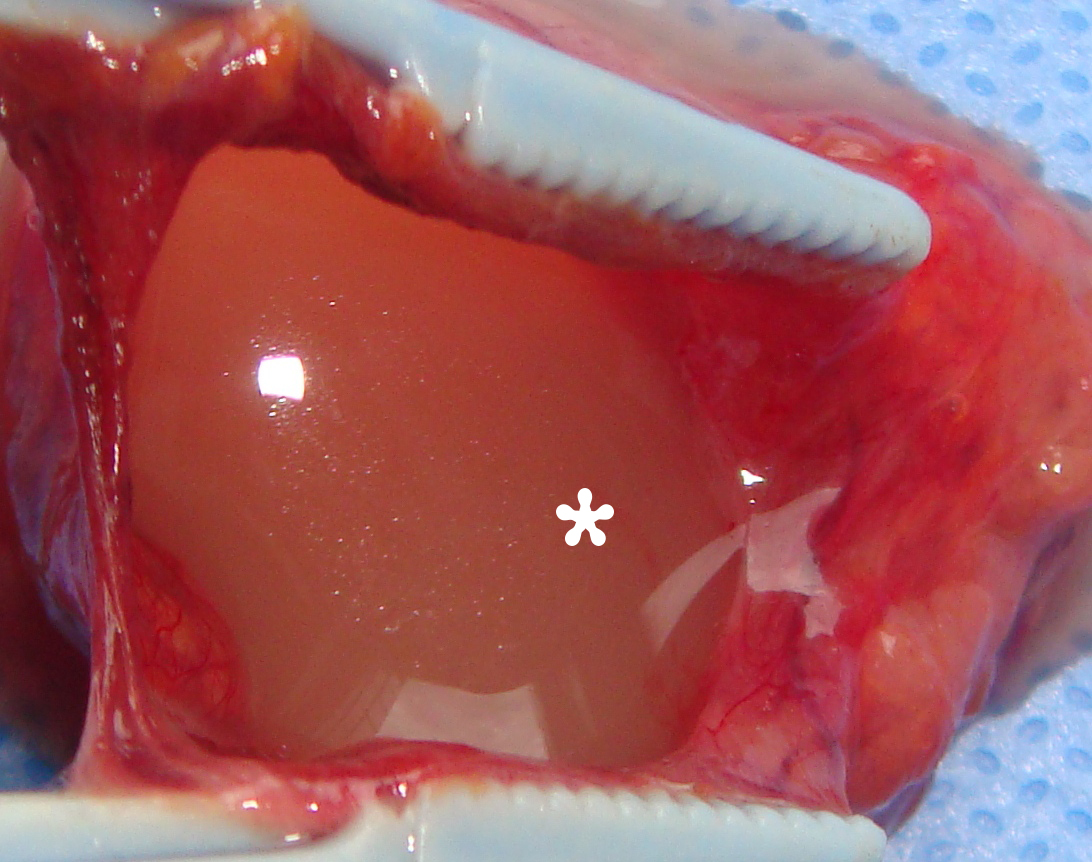
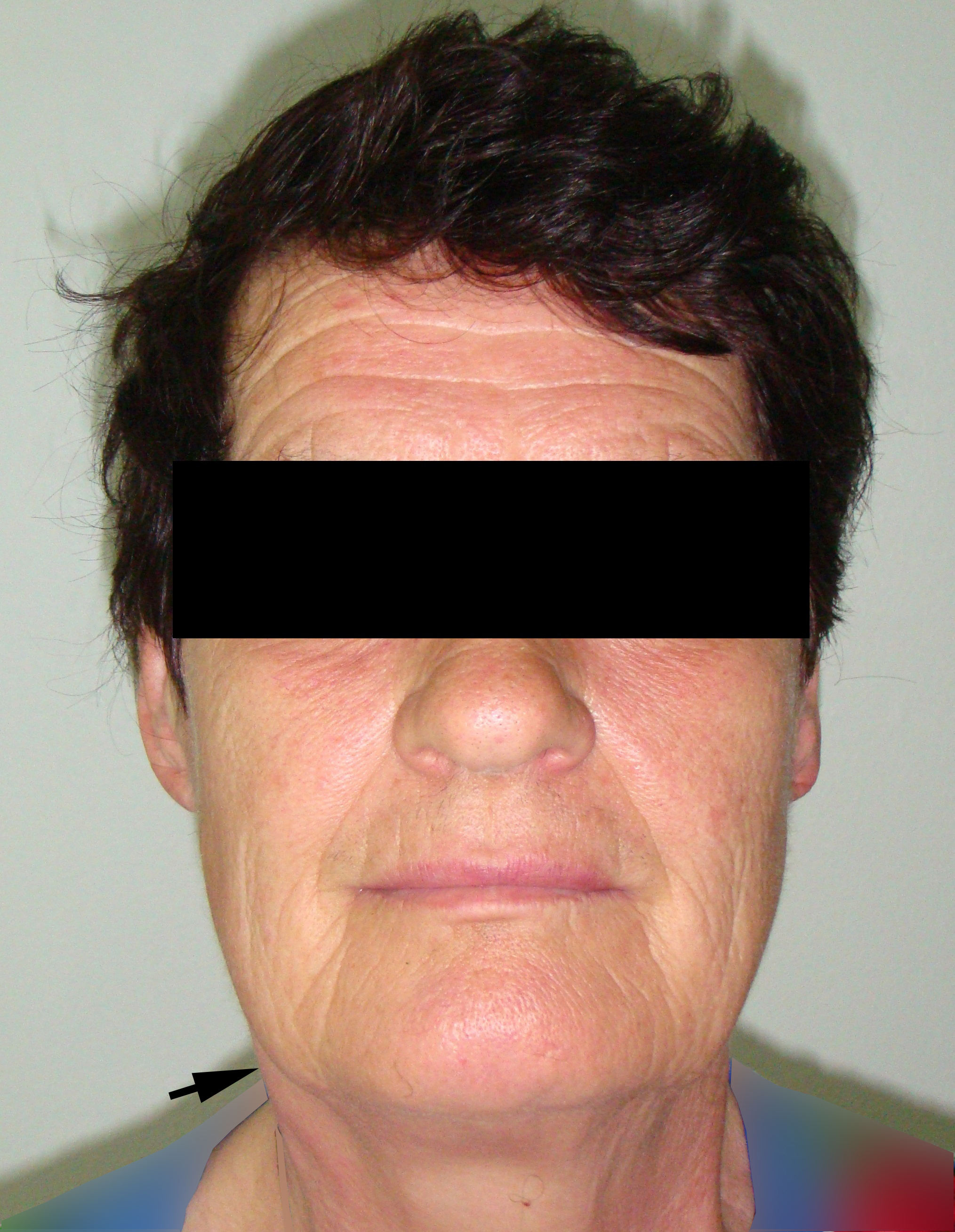
A 78-year-old Caucasian male was referred to our Department in June 2016 with soft tissue asymmetry in the left upper neck region (Fig 5). Gray-scale US showed presence of well-defined heterogenic parotid mass (Fig 6) which is visualized as multilocular cystic tumor (multiple anechoic areas with echogenic hair-like strands) due to the insignificant part of solid components. The long-to-short diameter of this tumor measured 3.44 × 1.62 cm. The tumor was elongated and rounded in shape at the edge of the mandible. Such US appearance was typical to Group 3 of Matsuda et al classification (2017)25 of anechoic area patterns and Grade 1 vascularization (flow signals were not noted). Tumor removal (with extracapsular dissection and facial nerve dissection) was done by V.H.D. under the general anesthesia. Cystic fluid was visualized after careful incision of one side of the Warthin’s tumor specimen (Fig 7A). Figure 7B demonstrates the part of aspirated fluid inside the 10.0-ml syringe. Histological diagnosis of adenolymphoma was established by pathologists (P.P.S. and V.I.Z.).
FIGURE 6. Case 4. A 78-year-old male patient. Preoperative longitudinal gray-scale sonograms (A, С) of the WT in the left parotid gland (PG). Tumor shows macrocystic appearance (CP, cystic part) with septations (arrowhead). Asterisk labels acoustic shadowing behind the superficial surface of the mandible (M). The long-to-short diameter (A) of this tumor measured 3.44 × 1.62 cm (calipers ‘+’ and ‘×’). B and D, position of the linear probe upon the US. Printed with permission and copyrights retained by O.S.C.
~HALF CYSTIC-HALF SOLID: MATSUDA ET AL GROUP 3A (CASE 5)
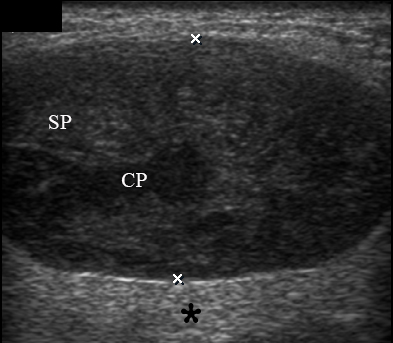
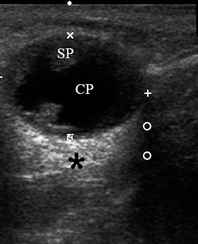
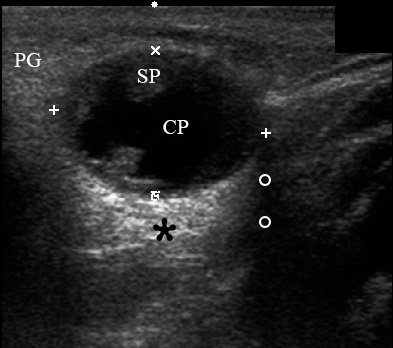
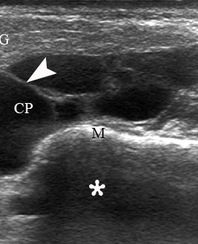
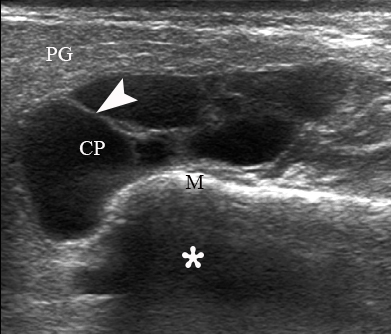
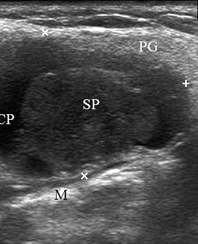
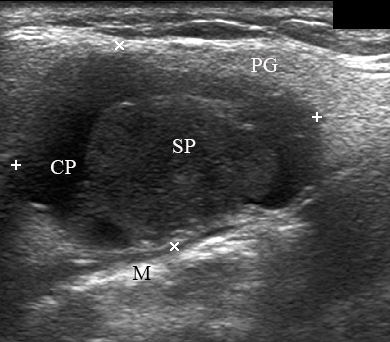
A 67-year-old Caucasian female was referred to our surgical department in June 2016 complaining for the existing painless mass in the right upper neck region (Fig 8). According to preoperative US (Fig 9), the long-to-short diameter of the tumor at B-mode was 2.69 × 1.84 cm. US appearance corresponds to Group 3 of Matsuda et al classification (2017)25 and Grade 2 vascularization (with three punctiform flow signals). Video (Supplemental Video Content) demonstrates longitudinal gray-scale ultrasonography of the Warthin’s tumor of the right parotid gland. Video is available in the page of the full-text article on www.dtjournal.org and in the YouTube channel, available at https://www.youtube.com/shorts/jKGX-t9rvv8.Total video’s duration: 22 sec. Tumor was removed by O.A.N. under the general anesthesia applying partial parotidectomy. Histologically, the diagnosis of adenolymphoma was established (P.P.S. and V.I.Z.).
FIGURE 9. Case 5. Preoperative US in a 67-year-old female patient. The long-to-short diameter of the TW at this B-mode sonogram (A) measured 2.69 × 1.84 cm (calipers ‘+’ and ‘×’). PG, parotid gland; SP, solid part of the WT; CP, cystic part of the WT; M, mandible. B, position of the linear probe upon B-mode US. Printed with permission and copyrights retained by O.S.C.
SCREENSHOT FROM VIDEO. Case 5. A 67-year-old female patient. Supplemental Video Content demonstrates longitudinal gray-scale ultrasonography of the Warthin’s tumor of the right parotid gland. Imaging clearly shows ~half anechoic-half echoic ultrasound appearance (i.e., ~half cystic-half solid structure) (Matsuda and colleagues’ Group 3, Subgroup A). PG, parotid gland; SP, solid part of the WT; CP, cystic part of the WT. The depth of gray scale ultrasonography is 4.0 cm. Video is available in the page of the full-text article on www.dtjournal.org and in the YouTube channel, available at https://www.youtube.com/shorts/jKGX-t9rvv8.
Total video’s duration: 22 seconds. The video is a three-second recording of gray scale ultrasound that is repeated several times for better visualization.
Printed with permission and copyrights retained by O.S.C.
VIDEO. Case 5. A 67-year-old female patient. Supplemental Video Content demonstrates longitudinal gray-scale ultrasonography of the Warthin’s tumor of the right parotid gland. Imaging clearly shows ~half anechoic-half echoic ultrasound appearance (i.e., ~half cystic-half solid structure) (Matsuda and colleagues’ Group 3, Subgroup A). PG, parotid gland; SP, solid part of the WT; CP, cystic part of the WT. The depth of gray scale ultrasonography is 4.0 cm. Video is available in the page of the full-text article on www.dtjournal.org and in the YouTube channel, available at https://www.youtube.com/shorts/jKGX-t9rvv8.
Total video’s duration: 22 seconds. The video is a three-second recording of gray scale ultrasound that is repeated several times for better visualization.
Printed with permission and copyrights retained by O.S.C.
DISCUSSION
Matsuda et al (2017)25 perfectly described four reasons why ultrasonography (US) is advantageous over other imaging technologies (computed tomography [CT],28 magnetic resonance imaging [MRI]) in case of parotid tumors. The reasons are: (1) simplicity, (2) low cost, (3) non-invasiveness, and (4) real-time images (sonograms).25
Moreover, similar to otolaryngology (Hoffman and Pagedar, 2018; Slough et al, 2019),29,30 US is increasingly becoming an integral part of an oral and maxillofacial surgery practice.31–34
To our knowledge and due to the English literature search, Neiman et al (1976) were the first ones who published the sonographic image of the WT.35 Gretchen A. W. Gooding (1980) presented the sonogram of his patient with the WT.36 Of course, the quality of the ultrasound images evolved significantly from the late 1970s, and in 2022, we can absorb all the advantages of the highly developed US machines. High resolution gray-scale sonograms, cine-loops, sonopalpation, color, and power Doppler is not a full list of options, radiologist and surgeons can used in their practice. Even the method of sonograms storage evolved from using a Polaroid® camera (Ishikawa et al, 1983)37 to digital archiving in every US machine and possibility to transfer and reproduce it at electronic devices (laptops, smartphones, and tablets). Sonograms of the WTs presented in the works from late 1970s and early 1980s clearly indicate us how far the evolution of the US had come from that moment.35–37,4 It is interesting that for almost all 46 years (since 1976 and Neiman's work), the constant tasks of radiologists and surgeons were attempts to describe the characteristic ultrasound pictures for parotid adenolymphoma.
Sriskandan and colleagues (2010) published the useful criteria for the assessment of ultrasonographic features of parotid and lymph node lesions.38 Knopf et al (2012) are also presented multimodal diagnostic pathway for unification of different ultrasonographic techniques for identification parotid WT, mixed tumor, and even carcinomas.22
In an own sonographic study, Miao et al (2015) divided pleomorphic adenomas and WTs into three groups: (1) without macroscopic cystic structures, (2) with <50 percent macroscopic cystic structures; and (3) with ≥50 percent macroscopic cystic structures.24 Matsuda and colleagues’ classification (2017) of the benign parotid tumors which is based on anechoic area patterns is more than worth of attention of oral and maxillofacial surgeons.25 The surgeons should understand the basic US terms. For example, anechoic area is black color area on the screen of the US machine or sonogram (Ihnatsenka and Boezaart, 2010),39 and is common for the fluid-filled areas of the tissues. Hyperechoic area―the white area on sonogram― is typical for bone structures (e.g., surface of the mandible).39 Dark gray areas―i.e., hypoechoic areas―are typical for adipose tissue, lymph nodes, cartilages, tumors, etc.39
In some cases, the ultrasound appearance of WT can mimic pleomorphic adenoma.38 For example, cystic WTs can be confused with simple cysts (Koenig et al, 2017).40
Sommer et al (1979) and Wu et al (2020) perfectly described ultrasound artifacts―from physics to clinics.41,42 Typical US artifacts visualized on the sonograms upon the WTs’ diagnostics in the parotid area in our patients were acoustic shadowing (behind the bony structures [mandible]), the edge artifact, and acoustic enhancement.
Ahuja et al (2007) showed three typical US appearances of the WT: (1) with cystic component, solid papillary portion, and thin septa (i.e., Matsuda Group 3A) (is similar to our case 5), (2) with multiple sponge-like anechoic areas (i.e., Matsuda Group 4) (is similar to our case 2), and (3) with large anechoic area (i.e., Matsuda Group 3).43
Totally cystic WTs can be note occasionally (Rhys, 2011),44 what is similar to our case 4 in which the WT mimicked a multicameral variant of the second branchial cleft cyst due to the similar location and ultrasound appearance (Tymofieiev et al, 2017)31.
Ultrasonographic appearance of сase 5 is similar to the case of a 70-year-old male patient with a WT in the right parotid gland from the study of Rong and colleagues (2014).45 WT visualized as oval-shaped mass with large anechoic areas (Matsuda Group 3).45
The literature report that typical/classic US appearance of intraparotid WT (Kamble et al, 2013)23 is a WT with the “~half cystic-half solid” structure.
Torske emphasized that cysts of the WT may contain yellow-brown fluid. This statement proved by case 4 (Figure 7A and B).46
Mantsopoulos et al (2018)6 presented B-mode sonograms of the WTs with multifocal growth (in a single organ) what is different from multicentric growth (in more than one organ) (de Werra and colleagues, 2009)47. The case of Shugar et al (1982) clearly showed evidence of three distinct WTs in every parotid gland (i.e., multicentric WT).48 The surgeons should be aware of possible synchronous unilateral parotid pleomorphic adenoma and WT as such case was presented by Heine et al (2018).49
Nascimento et al (2014) emphasized that WT can occur unilaterally or bilaterally, metachronously or synchronously.50 WT is multifocal in 20 percent. In rare cases, even the ulcerative WT can be obsereved.51
The male to female ration in our study (3:2) adheres to the internationally proved data in which it ranges from 2.6:1 to 10:1 (Chapnik, 1983; Teymoortash et al, 2006)4,7. The average age of the investigated patients was 65.4 years what is similar to the reported data of Torske who reported that 6th to 7th decades are the decades of WT manifestations.46
Figure 10 showed our supplement to the classification of Matsuda et al (2017).25
All diagnostic measures for parotid tumor processes are subject to one goal - to choose the most appropriate type of operation. Heller and Attie (1988) and Batori et al (2002) advocated for enucleation as a recommended surgery for adenolymphoma.52,53 Carlson and Ord (2008) emphasized on a need to perform partial parotidectomy as WTs have a tendency to occur in the caudal part of parotid.54 Tymofieiev (2012) emphasized, that it is impossible to determine accurately a parotid tumor type in advance when removing a tumor.8 It is advisable at least to perform a partial parotidectomy, or better, a subtotal parotidectomy with preservation of the facial nerve branches (Tymofieiev, 2012).8 Surgical possibilities (enucleation, parotidectomy) related with WT cases are also well described in the Iowa Head and Neck Protocols.55,56
Histologic subclassification of WTs was reported by Seifert et al (1980) based on their 275 WT cases.57 This subclassification includes typical WT (77 percent), stroma-poor WT (13.5 percent), stroma-rich WT (2 percent), and metaplastic WT (7.5 percent).57
Despite the fact that the terms adenolymphoma and Warthin’s tumor are predominantly applied for the description of this pathological parotid entity, we adhere to the term “cystadenolymphoma.” Based on the macroscopic structure of this tumor, the ultrasound appearance, and opinions of experts (Teymoortash et al, 2006;7 Mantsopoulos et al, 20186) we recommend to use cystadenolymphoma term.
We agree with Khatib et al (2022), who noticed that case series study is weaker comparing to clinical study with a big number of patients.58 But we hope that this article can be useful for other researchers in this topic and be a cornerstone for studies with bigger number of WT cases.
CONCLUSIONS
In conclusion, our supplement to Matsuda and colleagues’ classification25 of anechoic area patterns of the Warthin’s tumor can help surgeons around the globe to be more accurate in preoperative verification of cystadenolymphoma. This case series illustrate the growing importance of ultrasonography in the professional life of oral and maxillofacial and head and neck surgeons. Based on the cystic structure of this benign tumor and the ultrasound appearance presented in our case series, we propose to continue to use the term “cystadenolymphoma” with a purpose to emphasize the tumor`s structure.
TERM OF CONSENT
Writing patients’ consents were obtained for publication the photos.
AUTHOR CONTRIBUTIONS
Conceptualization: Cherniak OS. Ultrasonographic data acquisition: Cherniak OS, Savchuk LA, Ripolovska OV. Surgical images acquisition: Demidov VH, Nozhenko OA. Histological data acquisition: Snisarevskyi PP, Zaritska VI. Data analysis or interpretation: Cherniak OS, Zaritska VI. Drafting of the manuscript: Cherniak OS. Critical revision of the manuscript: Cherniak OS, Zaritska VI, Nozhenko OA. Approval of the final version of the manuscript: all authors.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
FUNDINGS
No funding was received for this study.
ACKNOWLEDGMENTS
We would like to thank Ievgen I. Fesenko, DSS, PhD from Kyiv (Ukraine) for the kind support in preparing the manuscript.
REFERENCES (58)
- Warthin AS. Papillary cystadenoma lymphomatosum. J Cancer Res 1929;13:116–25.
- Hildebrand O. About congenital epithelial cysts and fistulas of the neck [in German]. Über angeborne epitheliale Cysten und Fistelns des Halses. Arch F Klin Chir 1895;49:167–92.
- Albrecht H, Arzt L. Contributions to the question of tissue aberration. Papillary cystadenomas in lymph glands [in German]. Beiträge zur Frage der Gewebsverirrung. Papilläre Cystadenome in Lymphdrüsen. Frankfurt Z Path 1910;4:47–69.
- Chapnik JS. The controversy of Warthin's tumor. Laryngoscope 1983;93(6):695–16. Crossref
- Tymofieiev OO. Diseases of the salivary glands [in Ukrainian]. 1st ed. Lviv: VNTL-Klasyka; 2007:107–58.
- Mantsopoulos K, Koch M, Goncalves M, Iro H. Investigation of the surgical strategies for unilateral multifocal cystadenolymphomas of the parotid gland. Oral Oncol 2018;82:176–80. Crossref
- Teymoortash A, Krasnewicz Y, Werner JA. Clinical features of cystadenolymphoma (Warthin’s tumor) of the parotid gland: a retrospective comparative study of 96 cases. Oral Oncol 2006;42(6):569–73. Crossref
- Tymofieiev OO. Manual of Maxillofacial and Dental Surgery [in Russian]. 5th ed. Kyiv: Chervona Ruta-Turs; 2012:882–901.
- Kim C-H, Han S-I, Kim M-Y. Warthin’s tumor of the parotid gland: a case report. J Korean Assoc Oral Maxillofac Surg 2012;38(6):366–70. Crossref
- Hellquist H, Skalova A. Warthin tumour. In: Histopathology of the salivary glands. Hellquist H, Skalova A, editors. 1st ed. Berlin Heidelberg: Springer-Verlag; 2014:119–39. Crossref
- Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg 1999;120:834e40.
- Kawakami M, Ito K, Tanaka H, Hyo S. Warthin's tumor of the nasopharynx: a case report. Auris Nasus Larynx 2004;31(3):293–8. Crossref
- Jordan J, Babiński D, Sova J. Adenolymphoma (Warthin's tumor) of the larynx: coexistence with the bilateral laryngocele. Contribution to differential diagnosis with oncocytic papillary cystadenoma [in Polish]. Gruczolak limfatyczny (guz Warthina) krtani współistniejacy z obustronna torbiela kieszonki krtaniowej. Przyczynek do róznicowania z onkocytarnym brodawczakowatym gruczolakotorbielakiem. Otolaryngol Pol 1999;53(2):213–6.
- Hilton A, Pankhania M, Jose J. Recurrent Warthin's tumour of the larynx. Ann R Coll Surg Engl 2015;97(4):e54–6. Crossref
- Iwai T, Baba J, Murata S, Mitsudo K, Maegawa J, Nagahama K, Tohnai I. Warthin tumor arising from the minor salivary gland. J Craniofac Surg 2012;23(5):e374–6. Crossref
- Snyderman C, Johnson JT, Barnes EL. Extraparotid Warthin's tumor. Otolaryngol Head Neck Surg 1986;94(2):169–75. Crossref
- Yamada S, Matsuo T, Fujita S, Suyama K, Yamaguchi A, Mizuno A. Mucoepidermoid carcinoma arising in Warthin's tumor of the parotid gland. Pathol Int 2002;52(10):653–6. Crossref
- Yu C, Song Z, Xiao Z, Lin Q, Dong X. Mucoepidermoid carcinoma arising in Warthin’s tumor of the parotid gland: сlinicopathological characteristics and immunophenotypes. Sci Rep 2016;6:30149. Crossref
- Kim J, Kim EK, Park CS, Choi YS, Kim YH, Choi EC. Characteristic sonographic findings of Warthin's tumor in the parotid gland. J Clin Ultrasound 2004;32(2):78–81. Crossref
- Zajkowski P, Jakubowski W, Białek EJ, Wysocki M, Osmólski A, Serafin-Król M. Pleomorphic adenoma and adenolymphoma in ultrasonography. Eur J Ultrasound 2000;12(1):23–9. Crossref
- Yuan WH, Hsu HC, Chou YH, Hsueh HC, Tseng TK, Tiu CM. Gray-scale and color Doppler ultrasonographic features of pleomorphic adenoma and Warthin's tumor in major salivary glands. Clin Imaging 2009;33(5):348–53. Crossref
- Knopf A, Mansour N, Chaker A, Bas M, Stock K. Multimodal ultrasonographic characterisation of parotid gland lesions--a pilot study. Eur J Radiol 2012;81(11):3300–5. Crossref
- Kamble RC, Joshi AN, Mestry PJ. Ultrasound characterization of salivary lesions. Otorhinolaryngol Clin 2013;5(4):16–29. Crossref
- Miao LY, Xue H, Ge HY, Wang JR, Jia JW, Cui LG. Differentiation of pleomorphic adenoma and Warthin's tumour of the salivary gland: is long-to-short diameter ratio a useful parameter? Clin Radiol 2015;70(11):1212-9. https://doi.org/1010.1016/j.crad.2015.06.085
- Matsuda E, Fukuhara T, Donishi R, Kawamoto K, Hirooka Y, Takeuchi H. Usefulness of a novel ultrasonographic classification based on anechoic area patterns for differentiating Warthin tumors from pleomorphic adenomas of the parotid gland. Yonago Acta Med 2018;60(4):220–6. https://doi.org/10.24563/yam.2017.12.002
- Gleave EN. An alternative to superficial parotidectomy: extracapsular dissection. In: Norman JED, McGurk M, editors. Salivary glands. St. Louis: Mosby-Wolfe; 1995:165–72.
- Witt RL, Rassekh C. Gland-preserving surgery for benign neoplasms. In: Gillespie MB, Walvekar RR, Schaitkin BM, Eisele DW, editors. Gland-preserving salivary surgery: a problem-based approach. 1st ed. Cham: Springer International Publishing; 2018:147–58. Crossref
- Demidov VH, Rybak VA. Giant parotid pleomorphic adenoma. J Diagn Treat Oral Maxillofac Pathol 2020;4(3):62–3. Crossref
- Hoffman HT, Pagedar NA. Ultrasound-guided salivary gland techniques and interpretations. Atlas Oral Maxillofac Surg Clin North Am 2018;26(2):119–32. Crossref
- Slough CM, Kamani D, Randolph GW. In-office ultrasonographic evaluation of neck masses/thyroid nodules. Otolaryngol Clin North Am 2019;52(3):559–75. Crossref
- Tymofieiev OO, Fesenko II, Cherniak OS, Zaritska VI. Features of diagnostics, clinical course and treatment of the branchial cleft cysts. J Diagn Treat Oral Maxillofac Pathol 2017;1(1):15–31. Crossref
- Nguyen JQN, Calabrese CE, Heaphy KJ, Koudstaal MJ, Estroff JA, Resnick CM. Can Robin sequence be predicted from prenatal ultrasonography? J Oral Maxillofac Surg 2020;78(4):612-8. Crossref
- Tymofieiev OO, Ushko NO, Fesenko II, Tymofieiev OO, Yarifa MO, Cherniak OS. Suppurative mastoid lymphadenitis mimicking mastoiditis: a case report. J Korean Assoc Oral Maxillofac Surg 2021;47(5):398–402. Crossref
- Walter P, Ketoff S, Benichou L, Laurian LJ, Caillot A. Case-report of an Abrikossoff tumor of the temporomandibular joint Rapport de cas d'une tumeur d'Abrikossoff de l'articulation temporo-mandibulaire. J Stomatol Oral Maxillofac Surg 2022;S2468-7855(22)00102–1. [published online ahead of print, 2022 Apr 26] Crossref
- Neiman HL, Phillips JF, Jaques DA, Brown TL. Ultrasound of the parotid gland. J Clin Ultrasound 1976;4(1):11–3. Crossref
- Gooding GA. Gray scale ultrasound of the parotid gland. AJR Am J Roentgenol 1980;134(3):469–72. Crossref
- Ishikawa H, Ishii Y, Ono T, Makimoto K, Yamamoto K, Torizuka K. Evaluation of gray-scale ultrasonography in the investigation of oral and neck mass lesions. J Oral Maxillofac Surg 1983;41(12):775–81. Crossref
- Sriskandan N, Hannah A, Howlett DC. A study to evaluate the accuracy of ultrasound in the diagnosis of parotid lumps and to review the sonographic features of parotid lesions - results in 220 patients. Clin Radiol 2010;65(5):366–72. Crossref
- Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg 2010;4(3):55–62. Crossref
- Warthin tumor. In: Diagnostic imaging, diagnostic imaging: oral and maxillofacial. Koenig LJ, Tamimi D, Petrikowski CG, Perschbacher SE, Ruprecht A, Benson BW, Hatcher D, Potter BJ, Harnsberger HR, editors. Part II, 2nd ed. Philadelphia: Elsevier; 2017:760–63. Crossref
- Sommer FG, Filly RA, Minton MJ. Acoustic shadowing due to refractive and reflective effects. AJR Am J Roentgenol 1979;132(6):973–9. Crossref
- Wu WT, Chang KV, Hsu YC, Hsu PC, Ricci V, Özçakar L. Artifacts in musculoskeletal ultrasonography: from physics to clinics. Diagnostics (Basel) 2020;10(9):645. Crossref
- Ahuja AT. Section 11: head and neck: Warthin tumor. In: , editors. Diagnostic imaging: ultrasound. 1st ed. Utah: Amirsys; 2007:11:76–9.
- Rhys R. Ultrasound of the neck. In: Clinical ultrasound. Allan PL, Baxter GM, Weston MJ, editors. 3rd ed. : Churchill Livingstone; 2011:890–919. Crossref
- Rong X, Zhu Q, Ji H, Li J, Huang H. Differentiation of pleomorphic adenoma and Warthin's tumor of the parotid gland: ultrasonographic features. Acta Radiol 2014;55(10):1203–9. Crossref
- Torske KR. Benign neoplasms of the salivary glands. In: Head and neck pathology. Thompson SDR, Goldblum JR, editors. 2nd ed. Philadelphia: W.B. Saunders; 2013:244–66. Crossref
- de Werra C, Donzelli I, Perone M, Micco RD, Orabona G. Multifocal and multicentric tumors. In: Multiple primary malignancies. Updates in surgery. Renda A, editor. 1st ed. Milan: Springer-Verlag Italia; 2009:129–42. Crossref
- Shugar JM, Som PM, Biller HF. Warthin's tumor, a multifocal disease. Ann Otol Rhinol Laryngol 1982;91(3 Pt 1):246–9. Crossref
- Heine D, Zenk J, Psychogios G. Two case reports of synchronous unilateral pleomorphic adenoma and cystadenolymphoma of the parotid gland with literature review. J Ultrason 2018;18(75):369–73. Crossref
- Nascimento LA, Ferreira JA, Pio RB, Takano GH, Miziara HL. Synchronous bilateral Warthin tumors: a case report. Int Arch Otorhinolaryngol 2014;18(2):217–20. Crossref
- Hung CJ, Kang BH, Wang JS, Lin YS. Ulcerative Warthin tumor: a case report and review of the literature. Ear Nose Throat J 2022;101(5):332–5. Crossref
- Heller KS, Attie JN. Treatment of Warthin's tumor by enucleation. Am J Surg 1988;156(4):294–6. Crossref
- Batori M, Mariotta G, Giovannone G, Casella G, Casella MC. Warthin's tumor of parotid gland: treatment of a retroneural lesion by enucleation. Eur Rev Med Pharmacol Sci 2002;6(5):105–11.
- Carlson ER, Ord RA. Tumors of the parotid gland. In: Carlson ER, Ord RA. Textbook and color atlas of salivary gland pathology: diagnosis and management. 1st ed. Ames, Iowa: Wiley-Blackwell; 2008:171–98.
- Hoffman HT. Iowa head and neck protocols: Warthins tumor 'shell out' with facial nerve monitoring [document on the internet]; last modified on Oct 17, 2018 [cited 2022 Apr 01]. Available from: Link
- Hoffman HT. Iowa head and neck protocols: parotidectomy - case example: multiple Warthin’s tumor [document on the internet]; last modified on Aug 30, 2019 [cited 2022 Apr 01]. Available from: Link
- Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch A Pathol Anat Histol 1980;388(1):13–38. Crossref
- Khatib B, Cheng A, Sim F, Bray B, Patel A. Challenges with the jaw in a day technique. J Oral Maxillofac Surg 2020;78(10):1869.e1–10. Crossref