A Rare Odontogenic Subperiosteal Abscess That Involved Entire Lateral Aspect of the Mandibular Body and Medial Aspect to the Level of Mylohyoid Ridge: Ultrasound Examination
February 28, 2023
J Diagn Treat Oral Maxillofac Pathol 2023;7: 10–20.
doi: 10.23999/j.dtomp.2023.2.1
Under a Creative Commons license
HOW TO CITE THIS ARTICLE
Cherniak OS, Fesenko II. A rare odontogenic subperiosteal abscess that involved entire lateral aspect of the mandibular body and medial aspect to the level of mylohyoid ridge: ultrasound examination. J Diagn Treat Oral Maxillofac Pathol 2023;6(2):10–20. https://doi.org/10.23999/j.dtomp.2023.2.1
NATIONAL REPOSITORY OF ACADEMIC TEXTS
https://nrat.ukrintei.ua/en/searchdoc/2023U000248/
INSTITUTIONAL REPOSITORY
https://ir.kmu.edu.ua/handle/123456789/517
SUMMARY
Management of odontogenic subperiosteal abscess (SPA) is one of routine procedures at daily oral surgery and dental practice. Typically, SPA is manifested as a one-side-of-the-jaw collection of purulent material between bony surface and periosteum. In contrast to the data published in numerous literary sources, we present a case reported for the first time in the English-language literature. A 42-year-old male patient with odontogenic SPA that involved two surfaces (aspects) of the mandibular body—lateral and medial aspect to the level of mylohyoid ridge—and inferior margin of the mandible is highlighted. Also, this article is designed to educate oral and maxillofacial surgeons on ultrasonographic possibilities for diagnostics of perimandibular subperiosteal infection highlighting an extremely rare case in the field.
INTRODUCTION
Subperiosteal abscess (SPA) is a collection of purulent material between periosteum and bone surface.1 The odontogenic SPA is a common pathology at an oral surgeon’s practice in out-patient clinics. In the East European countries, the term acute purulent “periostitis” is also applied for the description of SPA.2 Despite the fact that the term “periostitis” is misnomer, it is generally accepted in some countries and is used to simplify the term of subperiosteal abscess and emphasize the localization of the inflammatory process in relation to the bone surface. And in the classifications of those countries the acute purulent “periostitis” (i.e., SPA) belongs to one of two forms of the acute “periostitis”—serous and purulent.2 In the acute form, “periostitis” occurs in 94–95 percent of cases, and in the chronic form, in 5–6 percent.3 Literature sources reported that SPA is localized between periosteum and jawbone in a buccal, palatal, or lingual aspects of the jaw.4 Other sources detail and provide data that SPA is localized on one side of the jaw, often affecting it from the vestibular surface (in 93.4 percent of patients).3
That is why we present to your attention a unique case of an odontogenic SPA that involved two surfaces (aspects) of the mandible—lateral and medial aspect of the mandibular body to the level of mylohyoid ridge—and inferior margin of the mandibular bone. To our knowledge it is a first ever reported in the English literature case with detailed clinical, sonographic, intraoperative, and postoperative presentation.
CASE
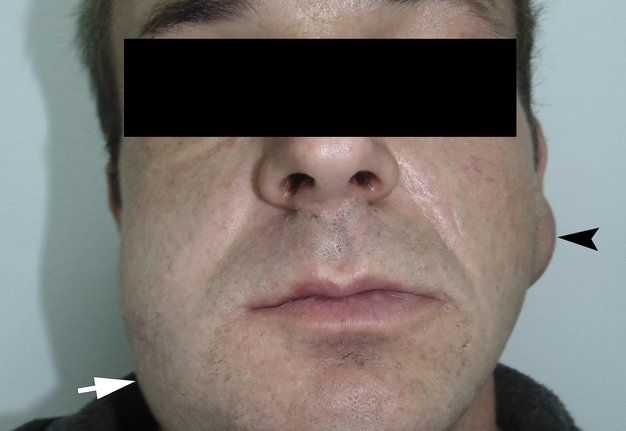
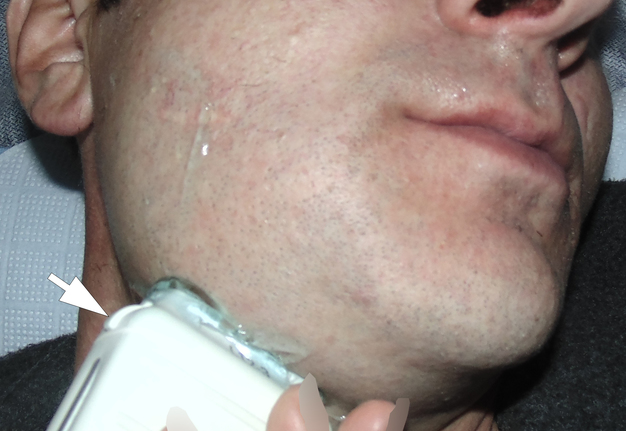
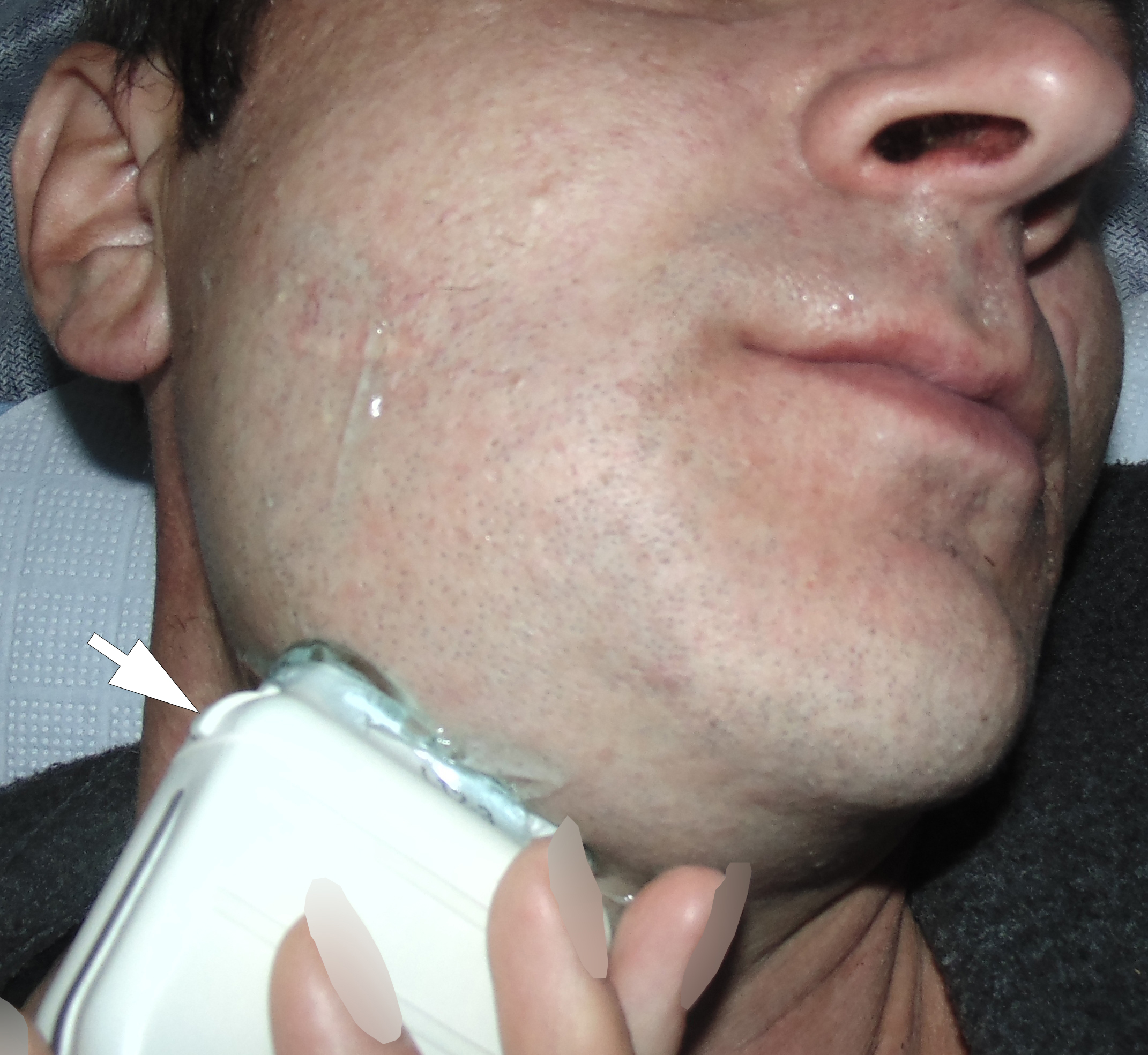
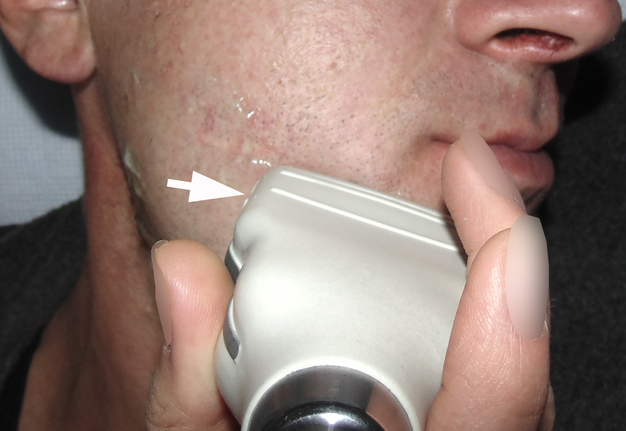
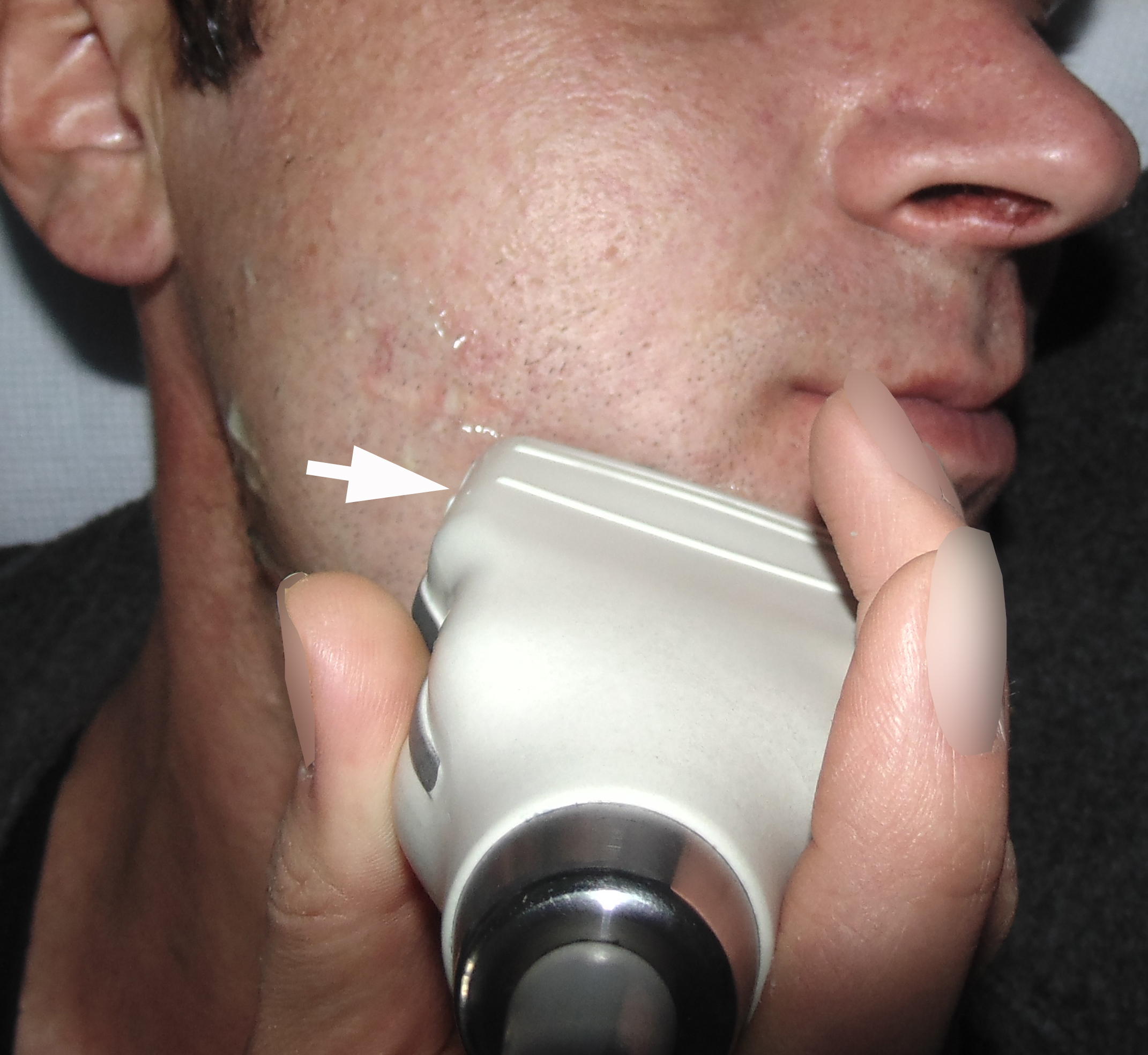
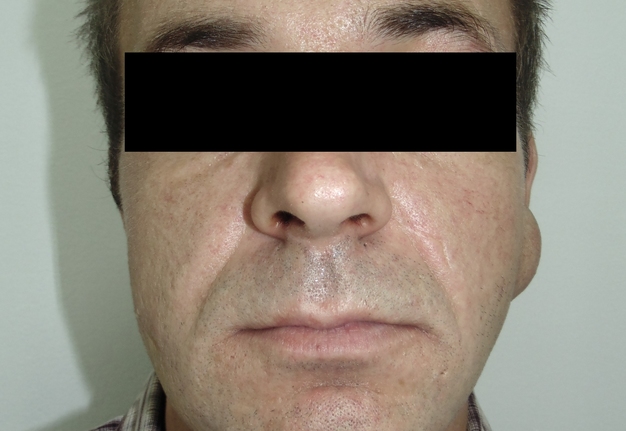
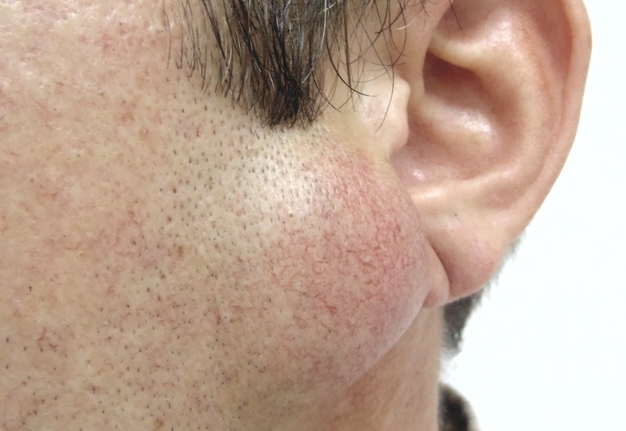
A 42-year-old male patient presented to the Center of Maxillofacial Surgery and Dentistry in 2015 with painful, increasing swelling in the right face (Fig 1) initiated after a several days pain in lower right first molar. The patient noted a worsened general condition, weakness, headache, fewer, and malaise. No comorbidities were reported by the patient.
Clinical examination revealed a swelling of soft tissues of the right cheek and submandibular area without cutaneous erythema. On palpation, a painful infiltrate was clearly noted along the periphery of the right mandibular body at lateral aspect, inferior margin, and at medial aspect. Intraorally, erythema of mucosa, painful swelling of the movable vestibular mucosa, and fluctuance were noted at the vestibular aspect of the mandibular body along the teeth 4.7, 4.6, 4.5, and 4.4. No evidence of erythema or similar swelling at the lingual aspect was noted. Teeth 4.6 and 4.5 showed insignificant mobility. Such clinical data as classic for odontogenic SPA edema and manifestations from the vestibular side and at the same time the presence of a painful infiltrate in the right mandibular area did not fit into the generally accepted diagnosis of SPA. Simultaneous swelling and infiltrate at the submandibular area was atypical for the SPAs.
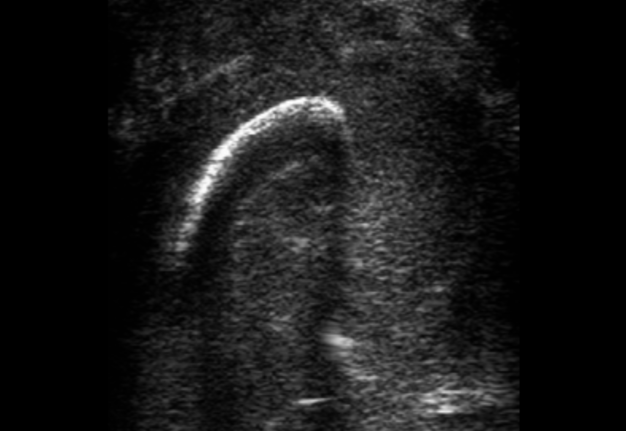
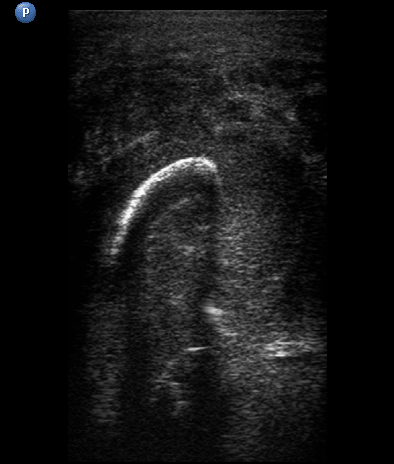
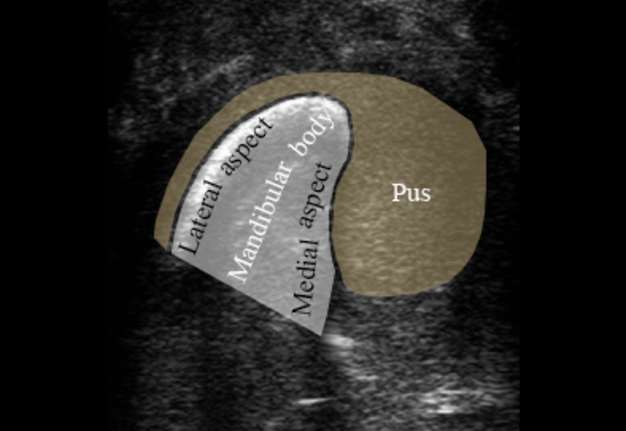
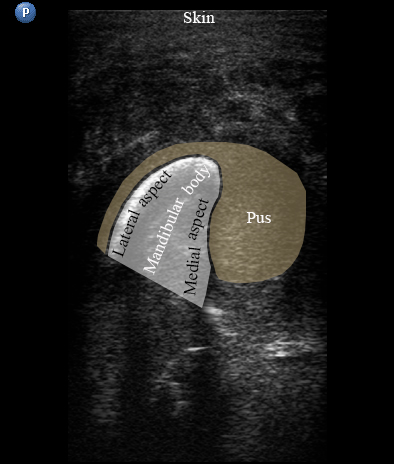
USG was performed on Philips ultrasound machine (model HD11 XE, Koninklijke Philips N.V.,Eindhoven, The Netherlands) by experienced doctor of ultrasound diagnostics (O.S.C., her experience in head and neck ultrasound is 16 years) using a 12-3 MHz linear probe (also termed as linear transducer). USG of the perimandibular tissues revealed collection of significant amounts of pus between elevated periosteum and bony surface around the lateral and medial aspects of the right mandible body (Figs 2 and 3). The purulent material visualized as hypoechoic, heterogeneous areawith internal debris and insignificant sign of fluid motion along the external and internal surfaces of the right mandibular body. Mandibular surfaces visualized as hyperechoic stripes with artifact of acoustic shadowing posteriorly. Collection of purulent material clearly visualized along the lateral (i.e., external) aspect the right mandibular body, inferior bony margin, and medial (i.e., internal) aspect. Worthy of attention is the fact that along the medial aspect of the mandible the pus reached the mylohyoid ridge, a place of attachment of the mylohyoid muscle. It can be said that medially the pus location was limited by subperiosteal space at the lower floor of mouth level.
FIGURE 2A. Transverse ultrasound scan of the right mandibular body (A). Arrow labels the probe’s bump that corresponds to the letter “P” (i.e., position) at the upper left corner of the sonograms and indicates on the probe’s side. B and C, Gray-scale sonograms. Noted collection of significant amounts of pus around the lateral and medial aspects of the mandible body. Prominent soft tissue oedema is also observed between skin and subperiosteal pus collection. The artifact of acoustic shadowing is noted posteriorly to the bony surface (hyperechoic curved stripe) of the mandible. The depth of ultrasonography is 6.0 cm. Printed with permission and copyrights retained by O.S.C. and I.I.F.
FIGURE 2B. Transverse ultrasound scan of the right mandibular body (A). Arrow labels the probe’s bump that corresponds to the letter “P” (i.e., position) at the upper left corner of the sonograms and indicates on the probe’s side. B and C, Gray-scale sonograms. Noted collection of significant amounts of pus around the lateral and medial aspects of the mandible body. Prominent soft tissue oedema is also observed between skin and subperiosteal pus collection. The artifact of acoustic shadowing is noted posteriorly to the bony surface (hyperechoic curved stripe) of the mandible. The depth of ultrasonography is 6.0 cm. Printed with permission and copyrights retained by O.S.C. and I.I.F.
FIGURE 2C. Transverse ultrasound scan of the right mandibular body (A). Arrow labels the probe’s bump that corresponds to the letter “P” (i.e., position) at the upper left corner of the sonograms and indicates on the probe’s side. B and C, Gray-scale sonograms. Noted collection of significant amounts of pus around the lateral and medial aspects of the mandible body. Prominent soft tissue oedema is also observed between skin and subperiosteal pus collection. The artifact of acoustic shadowing is noted posteriorly to the bony surface (hyperechoic curved stripe) of the mandible. The depth of ultrasonography is 6.0 cm. Printed with permission and copyrights retained by O.S.C. and I.I.F.
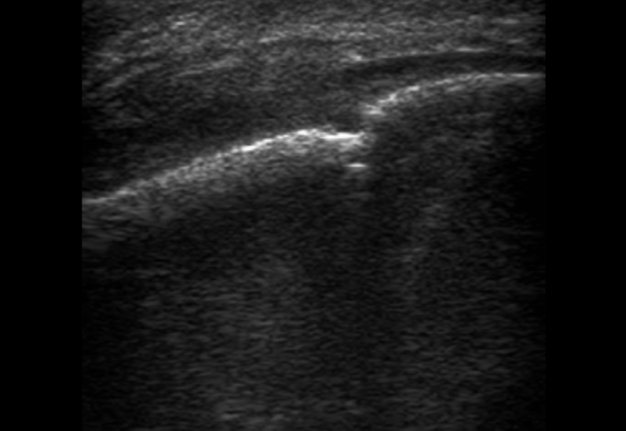
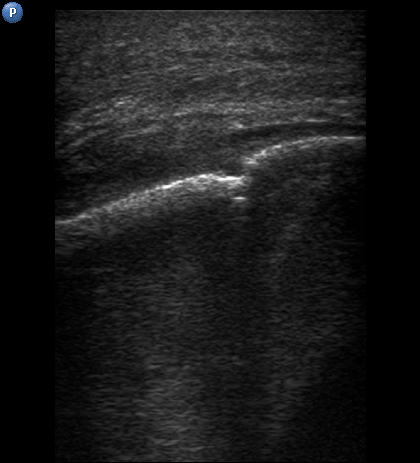
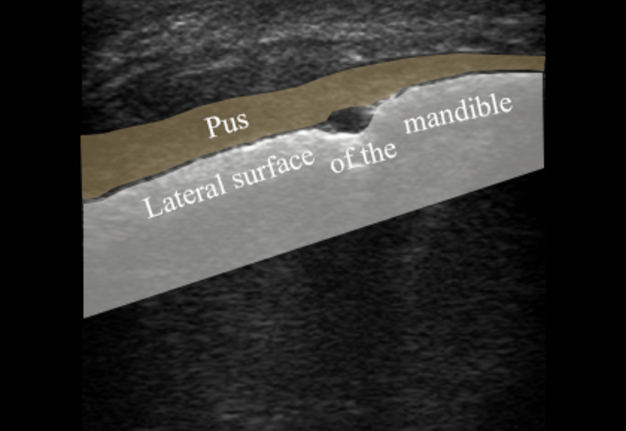
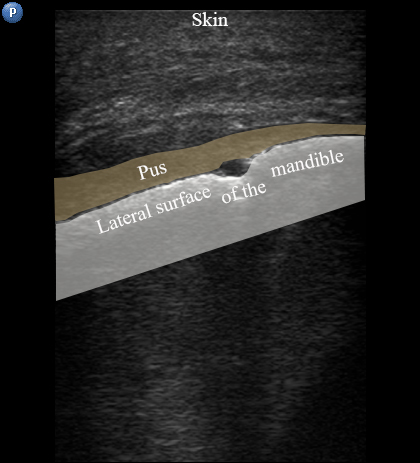
FIGURE 3A. Longitudinal ultrasound scan of the right mandibular body (A). Arrow labels the transducer’s bump that corresponds to the letter “P” (i.e., position) at the upper left corner of the sonograms and indicates on the transducer’s side. B and C, Gray-scale sonograms. Noted collection of significant amounts of pus along the lateral aspect (surface) of the mandible body. Prominent soft tissue oedema is also observed between skin and subperiosteal pus collection. The artifact of acoustic shadowing is noted posteriorly to the mandibular bone surface (hyperechoic stripe). The depth ofultrasonography presented at sonogram is 5.0 cm. Printed with permission and copyrights retained by O.S.C. and I.I.F.
FIGURE 3B. Longitudinal ultrasound scan of the right mandibular body (A). Arrow labels the transducer’s bump that corresponds to the letter “P” (i.e., position) at the upper left corner of the sonograms and indicates on the transducer’s side. B and C, Gray-scale sonograms. Noted collection of significant amounts of pus along the lateral aspect (surface) of the mandible body. Prominent soft tissue oedema is also observed between skin and subperiosteal pus collection. The artifact of acoustic shadowing is noted posteriorly to the mandibular bone surface (hyperechoic stripe). The depth ofultrasonography presented at sonogram is 5.0 cm. Printed with permission and copyrights retained by O.S.C. and I.I.F.
FIGURE 3C. Longitudinal ultrasound scan of the right mandibular body (A). Arrow labels the transducer’s bump that corresponds to the letter “P” (i.e., position) at the upper left corner of the sonograms and indicates on the transducer’s side. B and C, Gray-scale sonograms. Noted collection of significant amounts of pus along the lateral aspect (surface) of the mandible body. Prominent soft tissue oedema is also observed between skin and subperiosteal pus collection. The artifact of acoustic shadowing is noted posteriorly to the mandibular bone surface (hyperechoic stripe). The depth ofultrasonography presented at sonogram is 5.0 cm. Printed with permission and copyrights retained by O.S.C. and I.I.F.
Also, the extraoral examination revealed a presence of a soft, movable round-shape lesion at the left parotid area (Fig 1A). According to the patient’s words, the lesion existed last several years growing slowly without painful feelings. Patient stated that a symmetrical neoplasm on the opposite side existed several years ago until its removal was done in another institution. USG of the left parotid region showed avascular well-circumscribed round-shaped lesion measured 3.96 × 1.86 cm. Lesion showed an extra-parotid location, posterior acoustic enhancement, presence of posterior wall enhancement, and heterogenic content. Its sonographic features were described in detail in our previous paper.5
The diagnosis of odontogenic SPA that covered lateral and medial aspect of the mandibular body to the level of mylohyoid ridge was established. Based on panoramic radiography, intraoral examination, and patient’s words it was concluded that the causative tooth was the decayed lower right first molar. A concomitant diagnosis of acquired epidermal cyst of the left parotid area was also established.
Lancing of the SPA was done under the local potentiated anesthesia using 2.0 ml of Dexalgin® (Laboratorios Menarini SA, Barcelona, Spain) intramuscularly and 2.5 ml of Ultracaine® D-S forte (4% Articaine HCl injection with 1:100,000 epinephrine, Sanofi, Germany) intraorally.
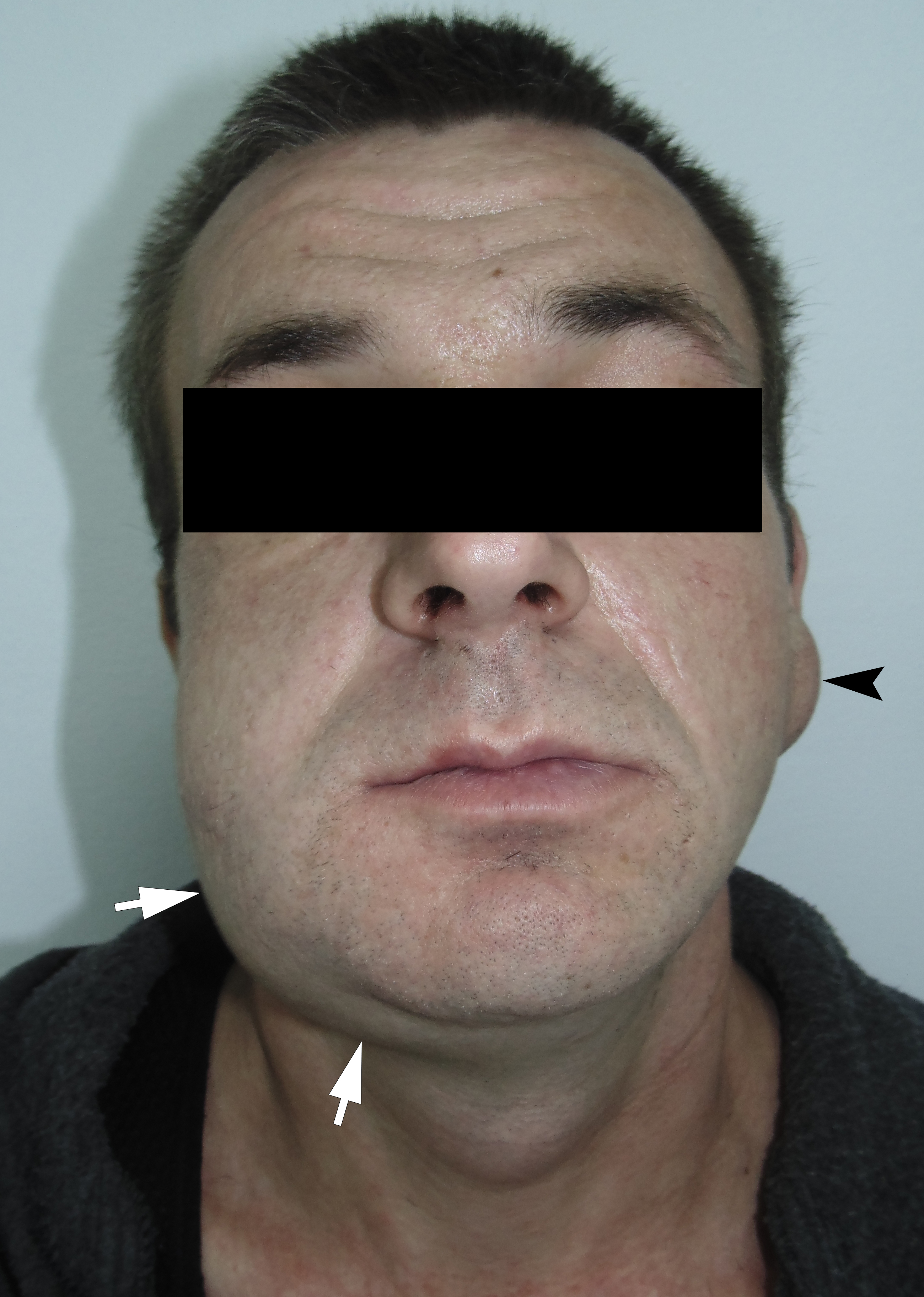
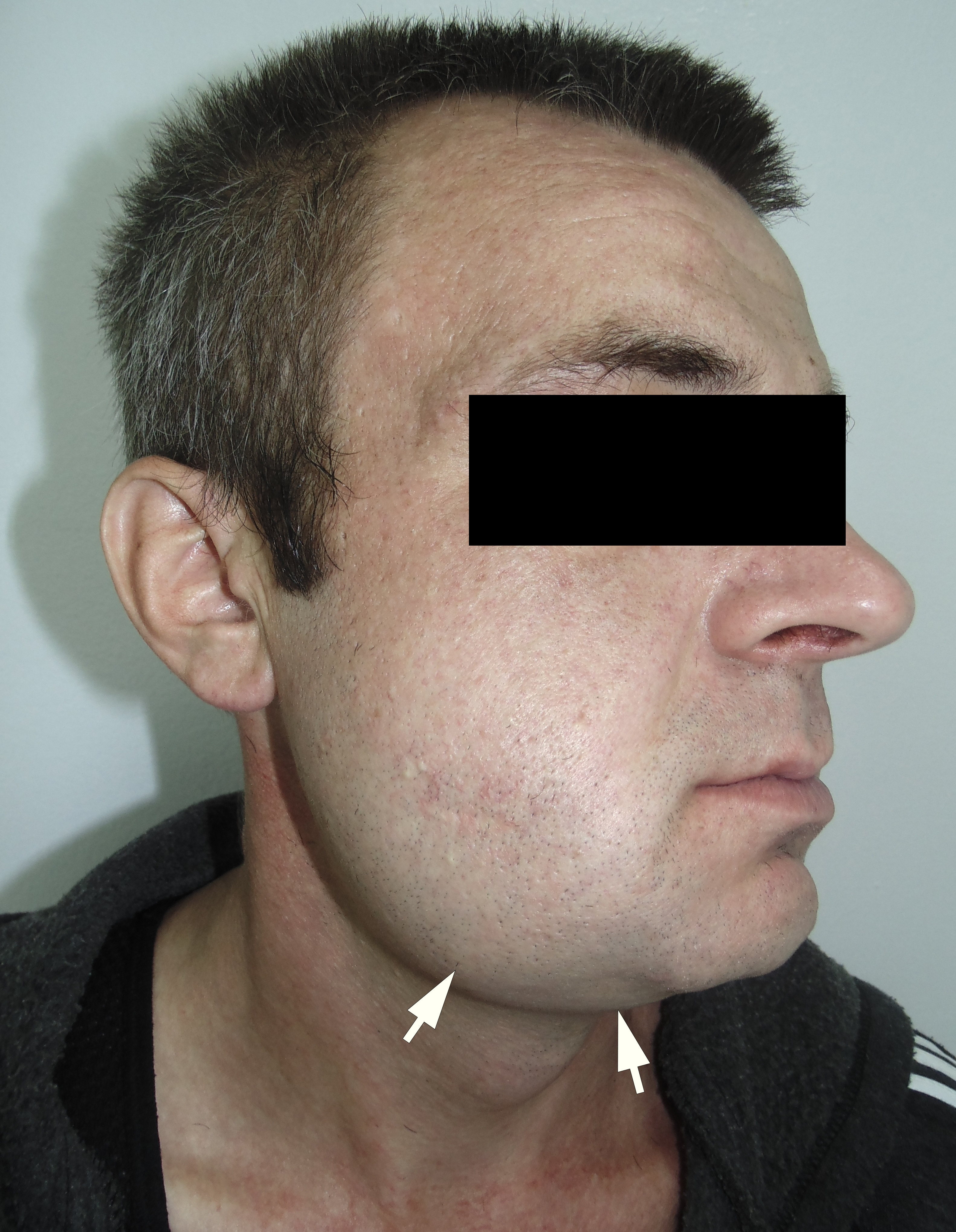
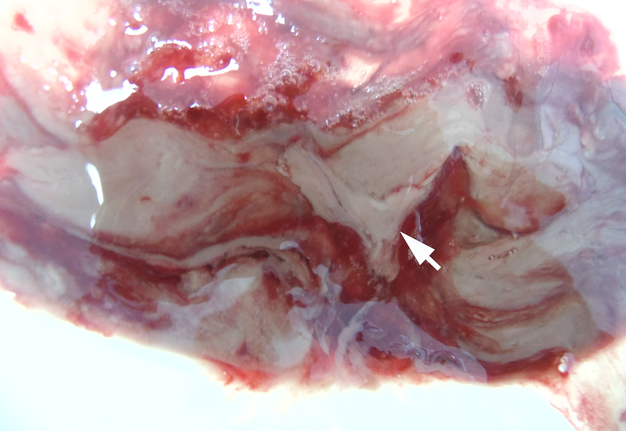
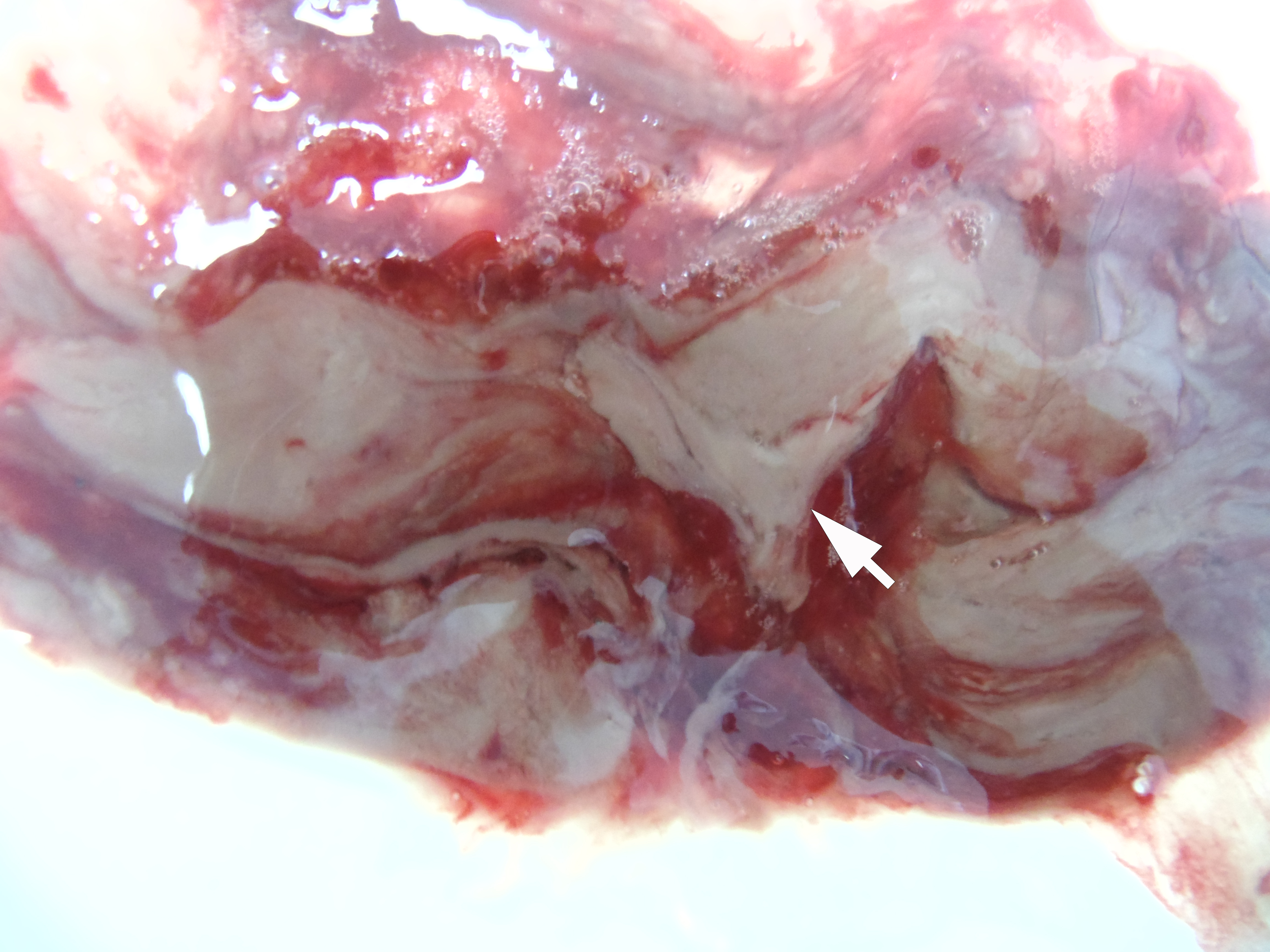
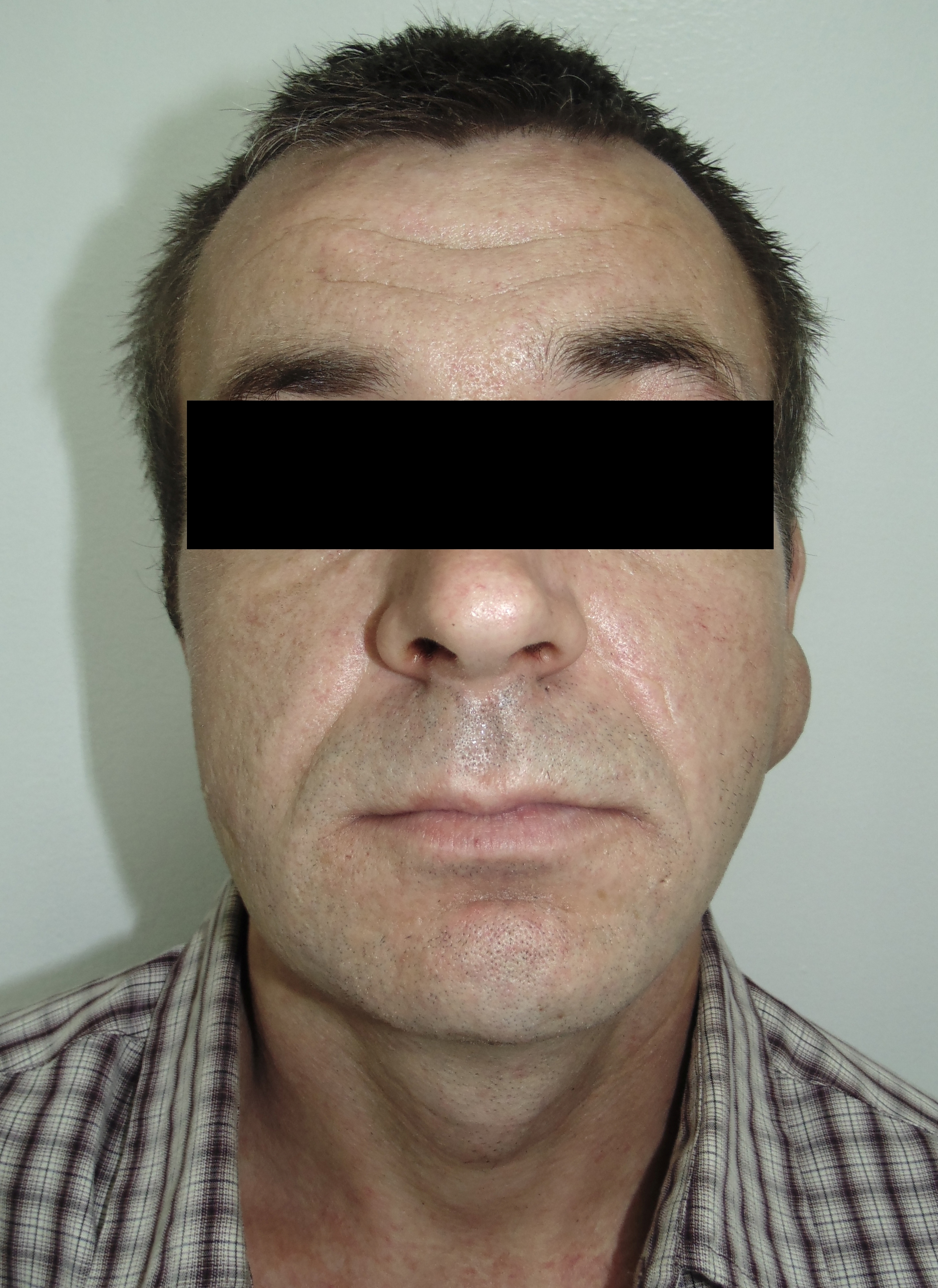
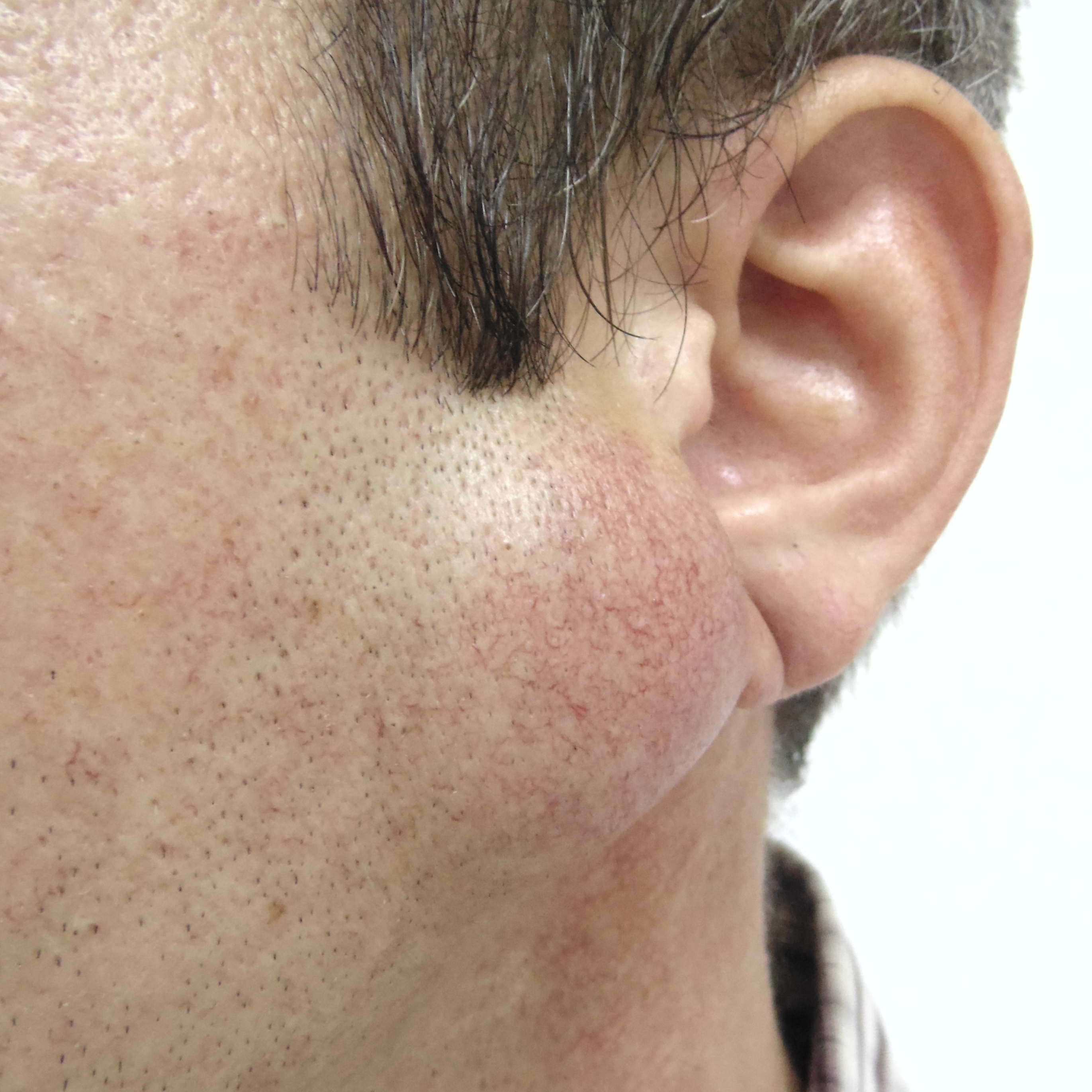
The incision was made along the teeth 4.7, 4.6, and 4.5 with blade no. 11, cutting the movable mucosa and periosteum to the bone. Approximately 16.0 ml of purulent material was evacuated via the wound. Pus evacuation (Fig 4) was stimulated by extraoral palpation of infiltrate at inferior margin and inner surface of the mandibular body. No incision of the mucosa and periosteum at the lingual aspect of the mandible was made. After that, a double perforated tubular drainage was used, self-made from a dropper system. It was inserted into the wound and installed slightly below the inferior margin of the mandible due to the presence of space between elevated periosteum and bony surface. Washing of the subperiosteal cavity through drainage was carried out upon the surgery and for 3 days with hospital solutions of the Furacilin and 0.02% Chlorhexidine. Then the drain was removed. Rinsing the mouth with an antiseptic solution has also been prescribed immediately after surgery. Suction of the wound was not performed. The patient refused to treat the tooth and insisted on its removal what was done at the day of SPA surgery. Postoperative recovery was smooth and uneventful. The edema decreased gradually over the next 6 days (Fig 5). A small amount of purulent content during antiseptic washing noted 3 days. No antibiotic therapy was prescribed due to good evacuation of the pus and fact that was purulent process limited by subperiosteal space only.
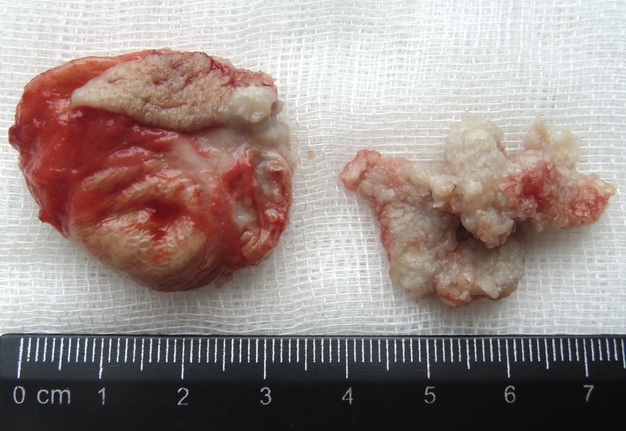
Removal of the cystic lesion in the left parotid area (Fig 6) was performed a week after the treatment of the SPA. The histopathological diagnosis confirmed the clinical and intraoperative diagnosis—acquired epidermal cyst (also known as punctum–associated cyst or formerly known as sebaceous gland cyst and atheroma).5,6
FIGURE 6A. A, Oblique view before the removal of cystic lesion in the left parotid area. B, Specimen of the cyst after the removal. Noted the typical punctum–associated cyst appearance.6 The histopathological diagnosis confirmed previous clinical diagnosis—acquired epidermal cyst.5 Printed with permission and copyrights retained by I.I.F.
FIGURE 6B. A, Oblique view before the removal of cystic lesion in the left parotid area. B, Specimen of the cyst after the removal. Noted the typical punctum–associated cyst appearance.6 The histopathological diagnosis confirmed previous clinical diagnosis—acquired epidermal cyst.5 Printed with permission and copyrights retained by I.I.F.
DISCUSSION
The diagnosis of SPA can be established in different anatomical locations.7–10 In case of odontogenic etiology of SPA the purulent content can be localized even in orbit.8,9 But the most common location of the odontogenic SPAs is a surface of the jaws.2–4 Literature reported that odontogenic SPA is typically involving one aspect (surface) of the jaw—vestibular or lingual/palatal.2–4,10
According to the data presented in 2012, in acute “periostitis” (serous or purulent [i.e., SPA]), the inflammatory process develops at vestibular aspect in 93.4 percent of patients.3 “Periostitis” proceeds in an acute serous form in 41.7 percent, in an acute purulent form in 58.3% percent.3 With purulent form of acute odontogenic “periostitis” (i.e., SPA), detachment of the periosteum over one tooth is noted in 20 percent of patients, over 2 teeth – in 56 percent, and over 3-4 teeth – in 24 percent.3
The case presented in this article is unique for the following reasons: (1) the fact that pus collected around the body of the mandible on both sides (lateral and medial aspects), seizing the inferior margin of the jaw, (2) successful application of the minimally invasive technique of one surgical approach and tubular double drainage to drain the abscess covered both body aspects, which is not typical for subperiosteal purulent processes, but such that allowed to effectively drain the wound and avoid additional incisions, and (3) according to recommendations3 the antibiotic therapy was not prescribed and the medication treatment was provided by the non-steroidal anti-inflammatory drug only. Literature emphasized that antibiotic therapy in odontogenic SPA cases is prescribed only for debilitated individuals or with concomitant diseases.3 This thesis was proved by the treatment of SPA in our case. Despite the fact that the process spread to two surfaces of mandibular body and a significant volume of purulent content, the use of correct surgical tactics and drug therapy, which included non-steroidal anti-inflammatory drugs and no antibiotics, managed to treat the patient successfully.
Location of the purulent material in the pus-created subperiosteal space was proved by high-resolution USG. Popularity of implementation of the USG into surgical practice continues to grow11–15 proving its role as a first-line imaging for head and neck pathology. A gray-scale USG in our case also proved its efficacy allowing us to make precise diagnosis which allowed to apply minimally invasive treatment and good outcome. It is important to emphasize that correct interpretation of USG in the floor of mouth and perimandibular areas is possible with good knowledge of anatomical structures and its interpretation on sonograms.16–19
In the 13 years of the surgeon’s practice (I.I.F), this is the first case of subperiosteal spread of purulent material on 3 surfaces from an odontogenic focus on the lower jaw. English literature search does not reveal the similar cases as well as its ultrasound descriptions.
Accompanying pathology in the parotid region is well described in relevant sources, both from the point of view of ultrasound diagnostics5,20 and surgical aspects2,3,6.
CONCLUSIONS
Diagnostic ultrasound proved its efficacy as a non-radiation imaging tool even for the rare odontogenic subperiosteal abscess cases which are involving lateral and medial aspects of the mandibular body. Application of ultrasonography allows precise diagnostic, treatment planning and surgical performance. Moreover, the high level imaging and surgical control of the purulent process allow to avoid antibiotics loading on patient’s body.
TERM OF CONSENT
Writing patient’s consent was obtained for publication the photos.
AUTHOR CONTRIBUTIONS
Conceptualization: Fesenko II, Cherniak OS. Ultrasonographic data acquisition: Cherniak OS. Surgical images acquisition: Fesenko II. Data analysis or interpretation: Cherniak OS, Fesenko II. Drafting of the manuscript: Fesenko II. Critical revision of the manuscript: Both authors. Approval of the final version of the manuscript: Both authors.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
FUNDINGS
No funding was received for this study.
REFERENCES (20)
-
Chandra RK, Kennedy DW. Sinus infection. In: Miloro M, Ghali GE, Larsen PE, Waite PD, editors. Peterson’s Principles of Oral and Maxillofacial Surgery. Hamillton: BC Decker Inc; 2004. p. 295–312.
-
Tymofieiev OO. Maxillofacial surgery. Kyiv: VSV “Medicine”; 2011. (in Ukrainian)
-
Tymofieiev OO. Manual of maxillofacial and oral surgery. Kyiv: Chervona Ruta-Turs LLC; 2012. (in Russian)
-
Fragiskos FD. Odontogenic infections. In: Fragiskos FD, editor. Oral surgery. Berlin Heidelberg: Springer-Verlag;2007. p. 205–41.
-
Tymofieiev OO, Ushko NO, Fesenko II, Cherniak OS. Maxillofacial surgery specialization in Ukraine: a new order and step in the growth of the specialty: analysis of qualification categories. J Diagn Treat Oral Maxillofac Pathol 2021;5(4):43–51. https://doi.org/10.23999/j.dtomp.2021.4.3
-
Fesenko II, Snisarevskyi PP, Zaritska VI. Infected punctum–associated cyst mimicking erysipelas. J Diagn Treat Oral Maxillofac Pathol 2021;5(2):15–9. https://doi.org/10.23999/j.dtomp.2021.2.1
-
Silva ACV, Lins CM, Mendes RFA, et al. Case report: Pott’s edematous tumor: complicated frontal sinusitis - an unremembered diagnosis. Front Surg 2022;9:889463. https://doi.org/10.3389/fsurg.2022.889463
-
Muñoz-Guerra MF, González-García R, Capote AL, et al. Subperiosteal abscess of the orbit: an unusual complication of the third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102(5):e9–e13. https://doi.org/10.1016/j.tripleo.2006.03.009
-
Geusens J, Dubron K, Meeus J, Spaey Y, Politis C. Subperiosteal orbital abscess from odontogenic origin: a case report. Int J Surg Case Rep 2020;73:263–7. https://doi.org/10.1016/j.ijscr.2020.07.014
-
Foskolos PG, Hadjipetrou S, Mylanos AI, Kolomvos N. Subperiosteal abscess: a postoperative complication after surgical extraction of impacted third molars. Report of a case. Hellenic Archives Oral Maxillofac Surg 2020;2:77–83.
-
Mallorie CN, Jones SD, Drage NA, Shepherd J. The reliability of high resolution ultrasound in the identification of pus collections in head and neck swellings. Int J Oral Maxillofac Surg 2012;41(2):252–5. https://doi.org/10.1016/j.ijom.2011.10.012
-
Mukhi PU, Mahindra UR. The use of ultrasonography in diagnosis and management of superficial fascial space infections. Indian J Dent Res 2012;23(3):313–9. https://doi.org/10.4103/0970-9290.102211
-
Cherniak OS, Ripolovska OV, Nozhenko OA, Fesenko II. Accuracy of ultrasound in diagnostics of odontogenic infection in layers of temporal and parotid masseter region. J Diagn Treat Oral Maxillofac Pathol2019;3(9):214−29. https://doi.org/10.23999/j.dtomp.2019.9.2
-
Torres-Gaya J, Boscà-Ramón A, Marqués-Mateo M, et al. Temporomandibular joint arthrocentesis guided by ultrasonography: an anatomical study. J Stomatol Oral Maxillofac Surg 2021;122(4):e27–e31. https://doi.org/10.1016/j.jormas.2021.03.002
-
Tymofieiev OO, Ushko NO, Fesenko II, et al. Suppurative mastoid lymphadenitis mimicking mastoiditis: a case report. J Korean Assoc Oral Maxillofac Surg 2021;47(5):398–402. https://doi.org/10.5125/jkaoms.2021.47.5.398
-
Madhok S, Kiruthika S, Prabhu K, et al. Mylohyoid ridge as a predictor of available bone for implant placement: acone-beam computed tomography (CBCT) retrospective observational study. Cureus 2022;14(7):e27470. https://doi.org/10.7759/cureus.27470
-
Kim SD, Loukas M. Anatomy and variations of digastric muscle. Anat Cell Biol 2019;52(1):1–11. https://doi.org/10.5115%2Facb.2019.52.1.1
-
La'porte SJ, Juttla JK, Lingam RK. Imaging the floor of the mouth and the sublingual space. Radiographics2011;31(5):1215–30. https://doi.org/10.1148/rg.315105062
-
Bell FE 3rd, Neuffer FH, Haddad TR, et al. Active learning of the floor of mouth anatomy with ultrasound. Anat Sci Educ 2019;12(3):310–6. https://doi.org/10.1002/ase.1839
-
Lee DH, Yoon CS, Lim BJ, et al. Ultrasound feature-based diagnostic model focusing on the “submarine sign” for epidermal cysts among superficial soft tissue lesions. Korean J Radiol 2019;20(10):1409–21. https://doi.org/10.3348/kjr.2019.0241