Accuracy of Ultrasound in Diagnostics of Odontogenic Infection in Layers of Temporal and Parotid Masseter Region
September 30, 2019
https://doi.org/10.23999/j.dtomp.2019.9.2
J Diagn Treat Oral Maxillofac Pathol 2020;4:214−29.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE
Cherniak OS, Ripolovska OV, Nozhenko OA, Fesenko II. Accuracy of ultrasound in diagnostics of odontogenic infection in layers of temporal and parotid masseter region. J Diagn Treat Oral Maxillofac Pathol 2019;3(9):214−29.
INSTITUTIONAL REPOSITORY
https://ir.kmu.edu.ua/handle/123456789/725
Contents: Summary | Introduction | Case | Discussion | Conflict Of Interest | Role Of The Authors | Fundings | Acknowledgements | References (40)
Summary
The current study presents the case of a first well described profound ultrasound (US) soft tissues examination in a 65-year-old female with odontogenic phlegmon of the masticator space. Consecutive preoperative clinical images, sonograms and US cine loops in comparison with asymptomatic side are presented and described. Terminology related with head and neck purulent conditions in the area of temporal and a masseter region is fundamentally analyzed.
INTRODUCTION
Purulent processes in the soft tissues of head and neck areas are often challenging even to experienced oral and maxillofacial surgeons. It is usually very disputable and unclear in what soft tissue layers a collection of pus is present.1 Knowledge of precise localization of purulent content is crucial for decisions regarding surgical intervention.1 There is a bunch of literary sources2-5 which describe diverse aspects of diagnostics and management of purulent head and neck conditions, but not enough number of articles6-8, presenting a profound description of ultrasound (US) images in those patients. And even many fewer studies focus on ultrasound upon infection of masticatory space.9,10 Despite the fact that many reports contain data about ultrasonography of masseter muscle in non-purulent cases,11,12 there is complete lack of studies, presenting good quality US scans with supplement video materials of infection in both parotid masseter and temporal region. We present a sequence of US images with three videos describing case of a 65-year-old woman with a severe odontogenic infection of temporal and parotid masseter regions. The purpose of our report is to cover the gap about US anatomy and investigation of masticatory space13 upon severe infection; the anatomical and nosological terminology will be discussed as well.
CASE
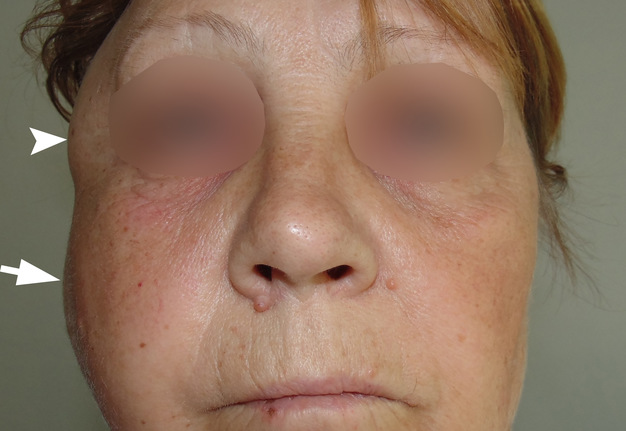
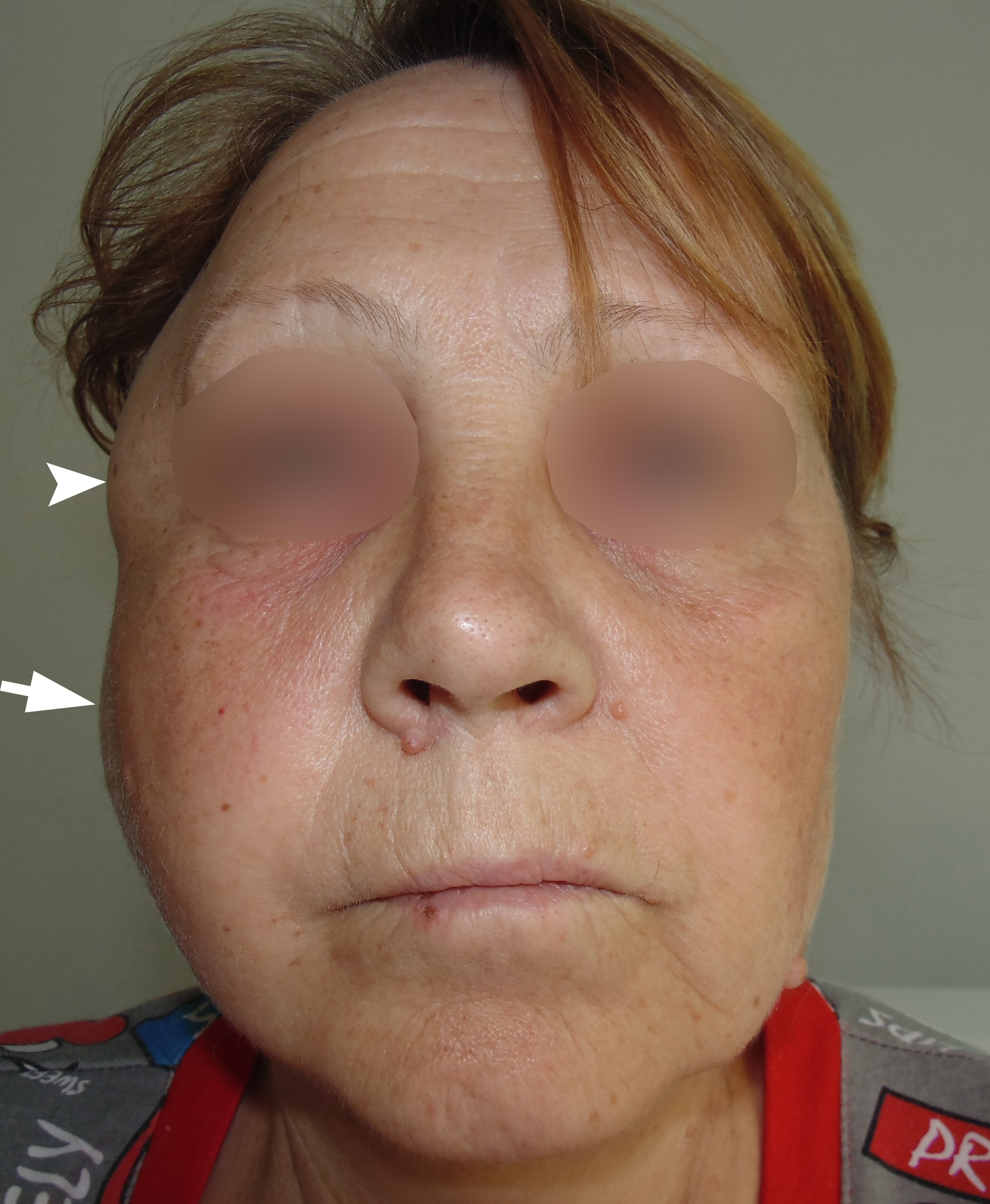
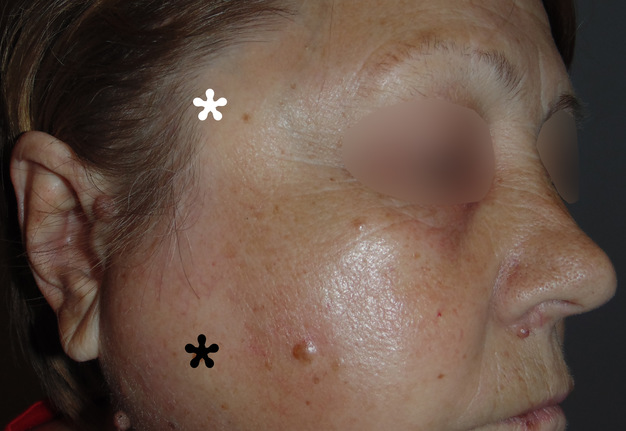
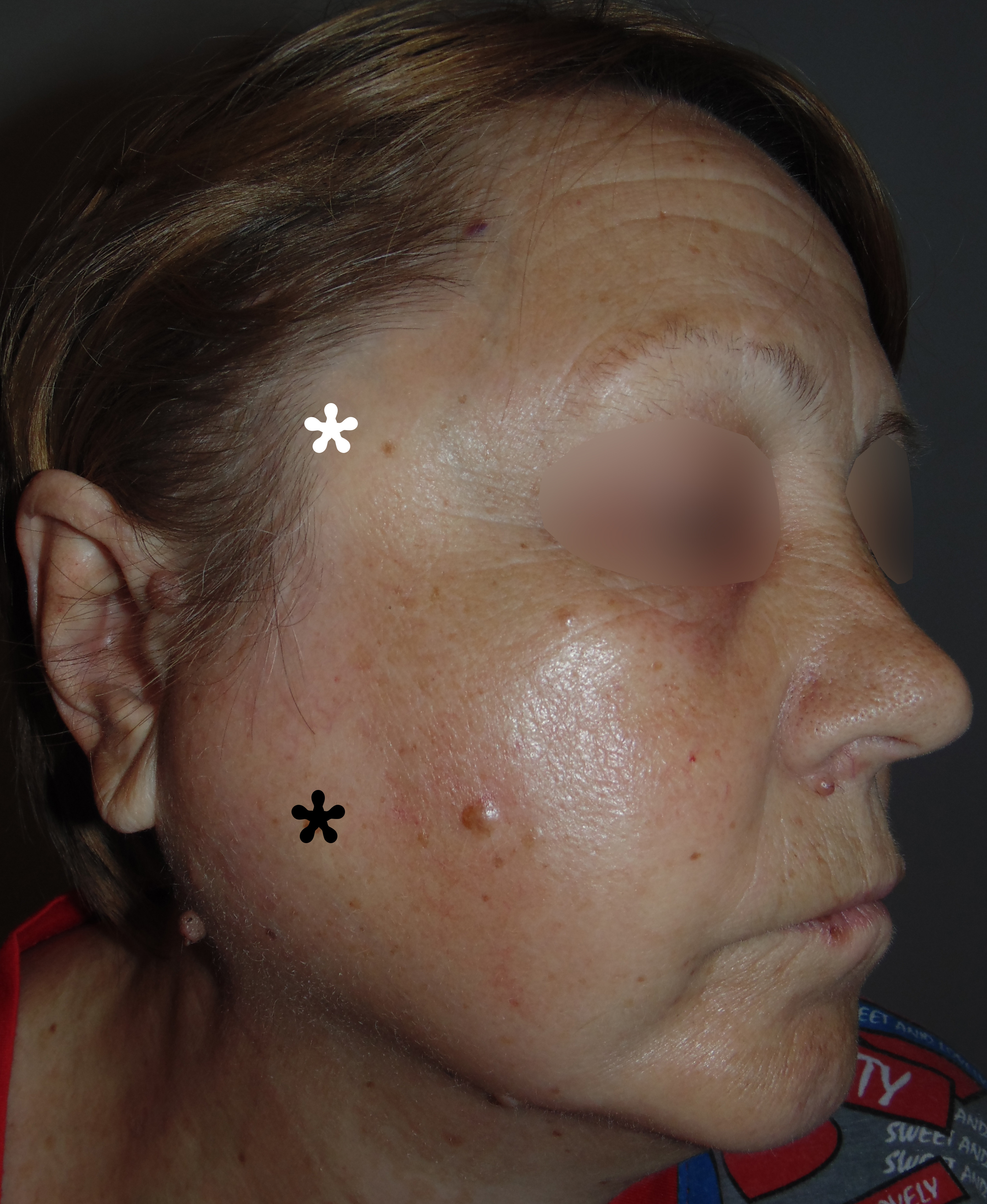
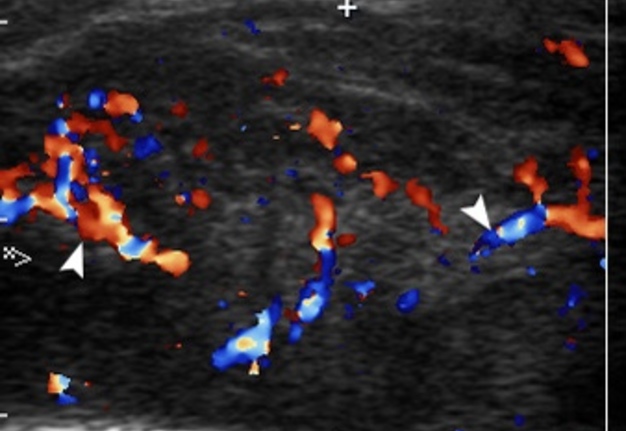
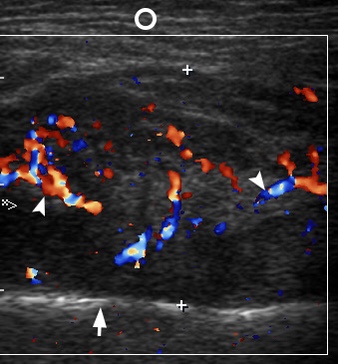
A 65-year-old female presented to the Kyiv Regional Clinical Hospital with 8-day history of increasing swelling first involved the right parotid masseter area and then also in right temporal area after a several day pain in the right lower second molar, severe trismus, fever, and orofacial pain. Clinical examination showed a notable right-side “hourglass” view (Figs 1 and 2) and palpation revealed painful tenderness in the swelled areas. Ultrasonography (model: HD11 XE, Philips) was performed by an experienced (12 years) physician of ultrasound diagnostics (O.S.C.) using linear transducer (12-3 MHz). For better understanding the soft tissue changes at sonograms of the areas of complaints we started US examination from the contralateral asymptomatic side. Gray scale sonograms of the left asymptomatic parotid masseter region (Fig 3) revealed no signs of inflammation: masseter muscle was 0.69 cm in thickness what was two times thinner than the inflamed contralateral masseter muscle. Seizes and echogenicity of the left parotid gland and subcutaneous cellular tissue were also not changed. There can usually be an assertion that it is worth measuring and comparing the size of the masseter muscle while relaxing the muscle and maximal occlusion.12 But in our case, as the patient had a trismus, the measurements on both sides were conducted in the same conditions. Sonograms of the left asymptomatic temporal region (Fig 4) showed no signs of inflammation in the soft tissues. B-mode US of the right masseter muscle (Fig 5) confirmed its enlargement up to 2.08 cm and subcutaneous cellular tissue (indicated by two ‘×’ calipers) – up to 0.8 cm. Dissociation of masseter fibers by hypoechoic fluid (pus) indicated for the surgeons that middle layer of masseter region have to be drained. Figure 6 (gray scale sonogram) shows a collection of significant amount of anechoic fluid, i.e., purulent content in our case, between outer surface of the mandibular ramus and fibers of masseter muscle as a typical location of submasseteric space abscess. This US data indicated to surgeons the fact that the deep layer of masseter region should also be drained. Color Doppler sonogram (Fig 7) of a right inflamed masseter muscle showed a striking increase of intramuscular vascularity, indicating the inflammatory hyperemia. Noted a significant swelling of subcutaneous cellular tissue and the masseter muscle was enlarged in two times (up to 2.2 cm). A collection of hypoechoic fluid (pus) between the muscle fibers is also indicated in Figure 7. The Videos 1-3 (Supplemental Video Content) clearly demonstrate location of purulent content in deep layers of masseter region, behind zygomatic arch, and temporal area. Videos are available in the page of the full-text article on dtjournal.org and in the YouTube channel ‘Videos DTJournal’, available at Video 1, Video 2, Video 3. Total duration of every video is 03 sec. Thus, US data gave to the surgeons a clear information that purulent content is located 1) between masseter muscle fibers, 2) between external surface of the ramus and masseter muscle, 3) involved fat pad of the temporal fascia, 4) fat pad deep to temporal fascia, 5) dissociating fibers and tendons of the temporal muscle, and reached 6) space between temporalis muscle and pericranium. According to Flynn anatomic spaces gradation of infection process severity, our case, with partial masticatory space involvement, related to moderate severity.14 The surgery was done under general anesthesia with fixation of double perforated tubular drainage systems for 3 days (with every day irrigation by antiseptic solution: 0.02% chlorhexidine hydrochloride). All locations in which the pus is visualized upon US have been confirmed presenting during the surgery.
DISCUSSION
First, before analyzing our case, we need to thoroughly discuss the terminology of purulent processes in head and neck areas accepted in different scientific works and countries. Five main terms, related with purulent inflammation in soft tissue layers, are very common in English-language sources: 1) cellulitis (synonym: inflammatory infiltrate), 2) abscess, 3) phlegmon, 4) necrotizing fasciitis, and 5) purulent-necrotic phlegmon.2
FIGURE 1. Anterior view of patient with diagnosis odontogenic phlegmon of the right parotid masseter and temporal regions before treatment. A significant swelling in the right temporal (arrowhead) and parotid masseter region (arrow) is representing an “hourglass” view.2 Printed with permission and copyrights retained by O.A.N.
FIGURE 2. Lateral view demonstrates swelling in the right temporal (white asterisk) and parotid masseter region (black asterisk) with no signs of skin redness, which indicates that purulent content localized in the deep layers of soft tissues. Printed with permission and copyrights retained by O.A.N.
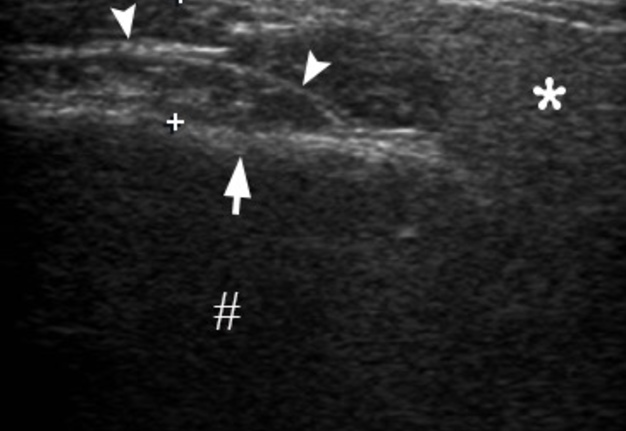
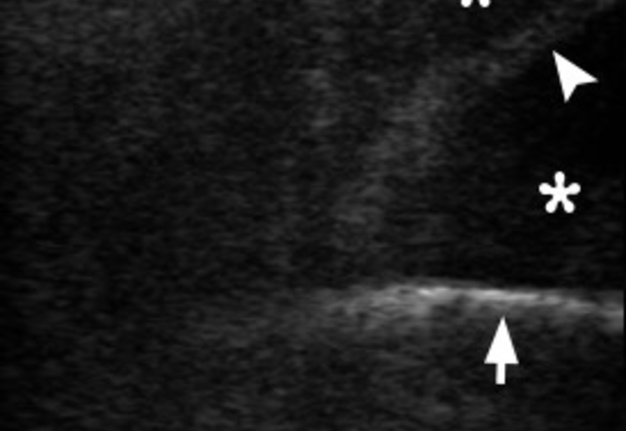
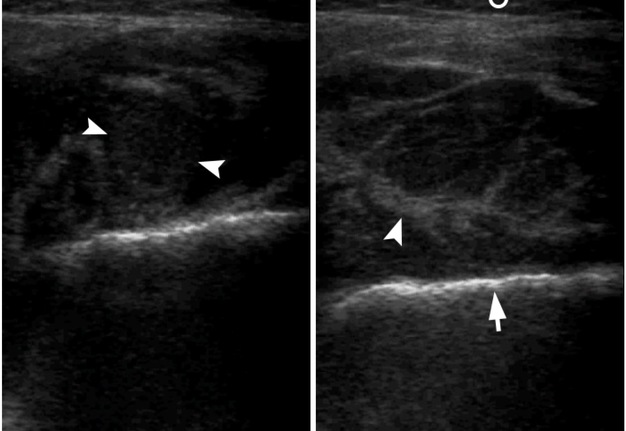
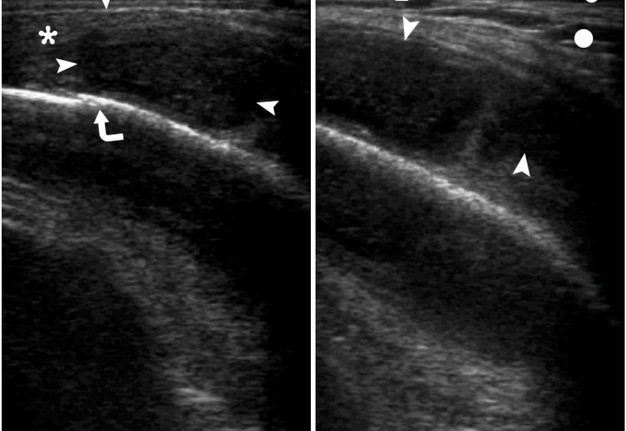
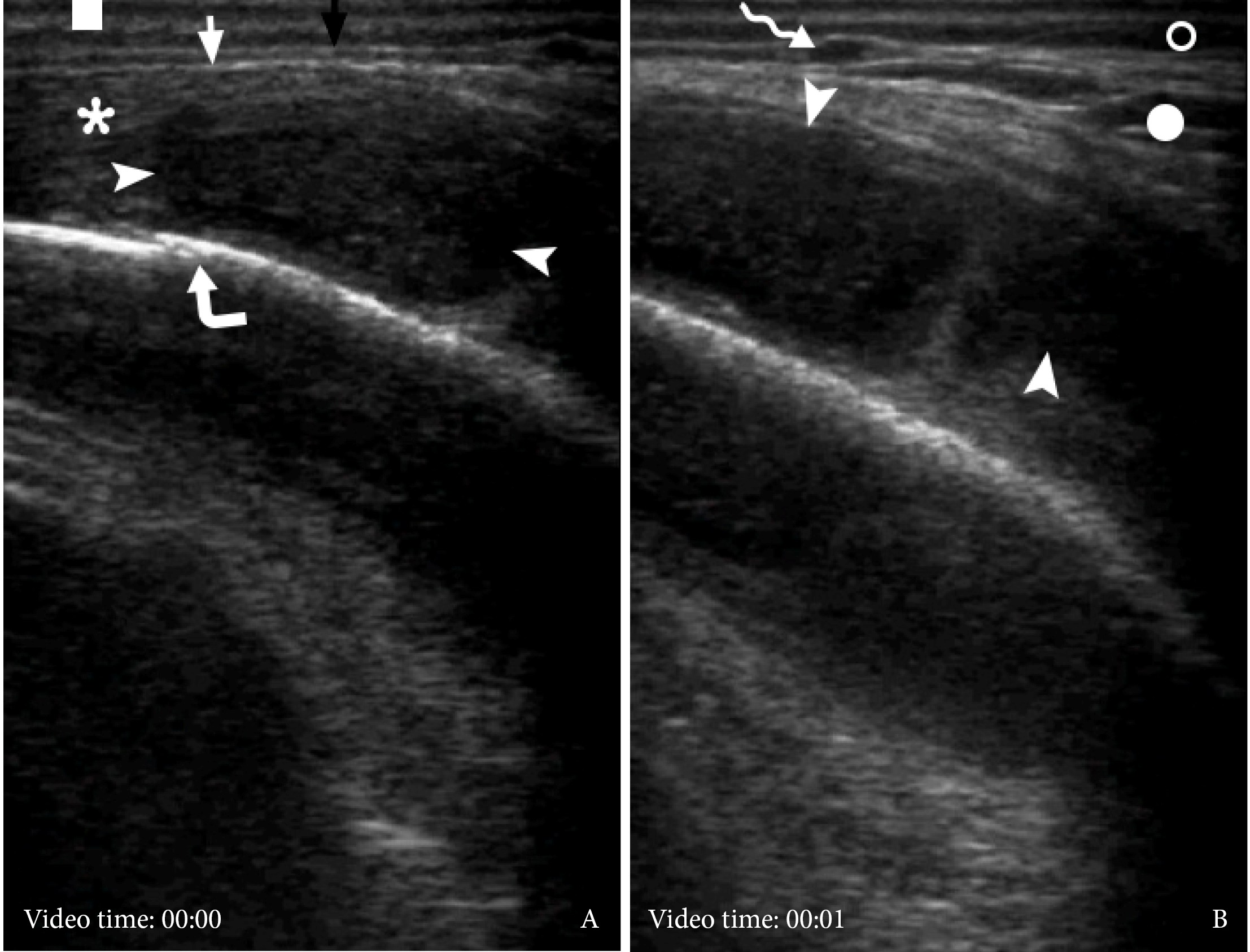
FIGURE 3. Transverse gray scale sonogram of the left asymptomatic parotid masseter region. Notes no signs of inflammatory process: masseter muscle (between ‘+’ calipers) is 0.69 cm in thick and it`s two times thinner than the inflamed contralateral masseter muscle. Connective tissue membranes between masseter sections and layers are indicated by arrowheads. Seizes and echogenicity of the left parotid gland (asterisk) and subcutaneous adipofascial tissue (circle) are not changed. Thick hyperechoic line (arrow) represents external surface of the mandibular ramus. Hashtag indicates artifact of acoustic shadowing, which is a result of reflection of US waves from the mandible and parotid fascia labeled by curved arrow. Printed with permission and copyrights retained by O.S.C. and I.I.F.
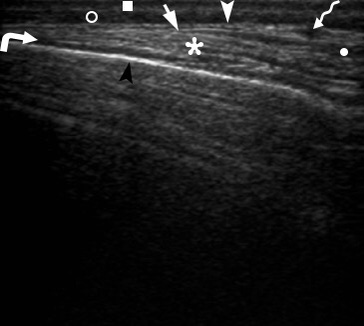
FIGURE 4. Oblique gray scale sonogram of the left asymptomatic temporal region shows no signs of inflammation in subcutaneous adipose tissue (open circle) or in other layers. Epidermis and derma are indicated by quadrate, tempoparietal fascia is indicated by white arrowhead, lumen of the vessel – by waved arrow, place of split of temporal fascia – by arrow, fat pad of temporal fascia – by small closed circle, tendons of temporal muscle – by asterisk, cellular tissue beneath temporalis – by curved arrow, outer surface of the bone of the lateral side of the skull – by black arrowhead. Printed with permission and copyrights retained by O.S.C. and I.I.F.
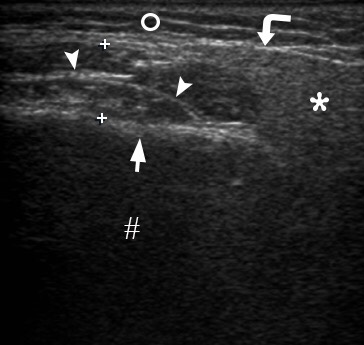
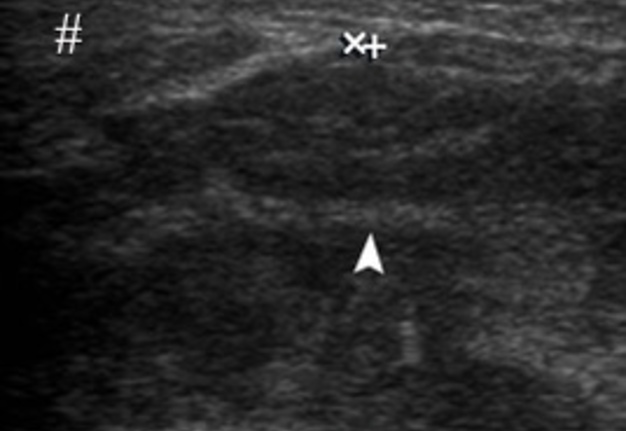
FIGURE 5. Transverse gray scale ultrasound demonstrates a swelling of the right masseter muscle (is indicated by two ‘+’ calipers) up to 2.08 cm and subcutaneous cellular tissue (is indicated by circle) up to 0.8 cm. Notes dissociation of masseter fibers (arrowhead) by small amounts of hypoechoic fluid (purulent content). Outer surface of the mandibular ramus is indicated by arrow and parotid gland – by hashtag. Printed with permission and copyrights retained by O.S.C. and I.I.F.
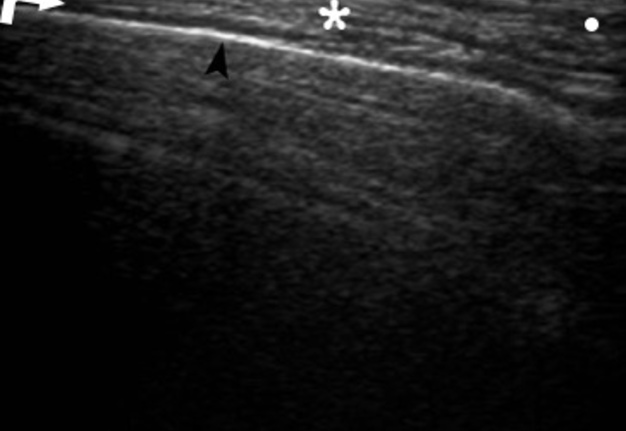
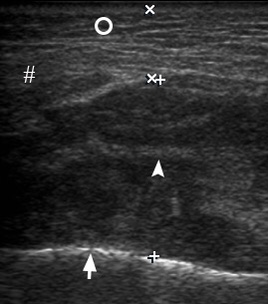
FIGURE 6. This transverse gray scale sonogram shows a collection of significant amount of anechoic fluid (asterisks), i.e., purulent content, between outer surface of the mandibular ramus (arrow), fibers of masseter muscle (arrowhead), right parotid gland (hashtag) which displaced by a collected pus. Subcutaneous tissue is labeled by circle. Printed with permission and copyrights retained by O.S.C. and I.I.F.
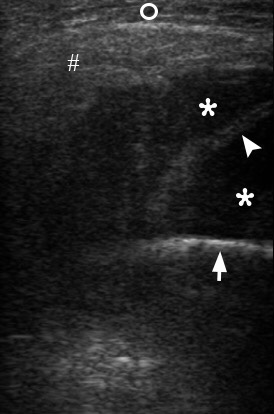
FIGURE 7. Transverse color Doppler sonogram shows a right inflamed masseter muscle (is indicated by two ‘+’ calipers) with a striking increase of intramuscular vascularity (arrowheads), which indicates about inflammatory hyperemia. Notes a significant swelling of subcutaneous adipofascial tissue (circle) and the masseter muscle is enlarged in two times (up to 2.2 cm). Notes a collection of hypoechoic fluid between the muscle fibers. Arrow indicates outer surface of the mandibular ramus. Printed with permission and copyrights retained by O.S.C. and I.I.F.
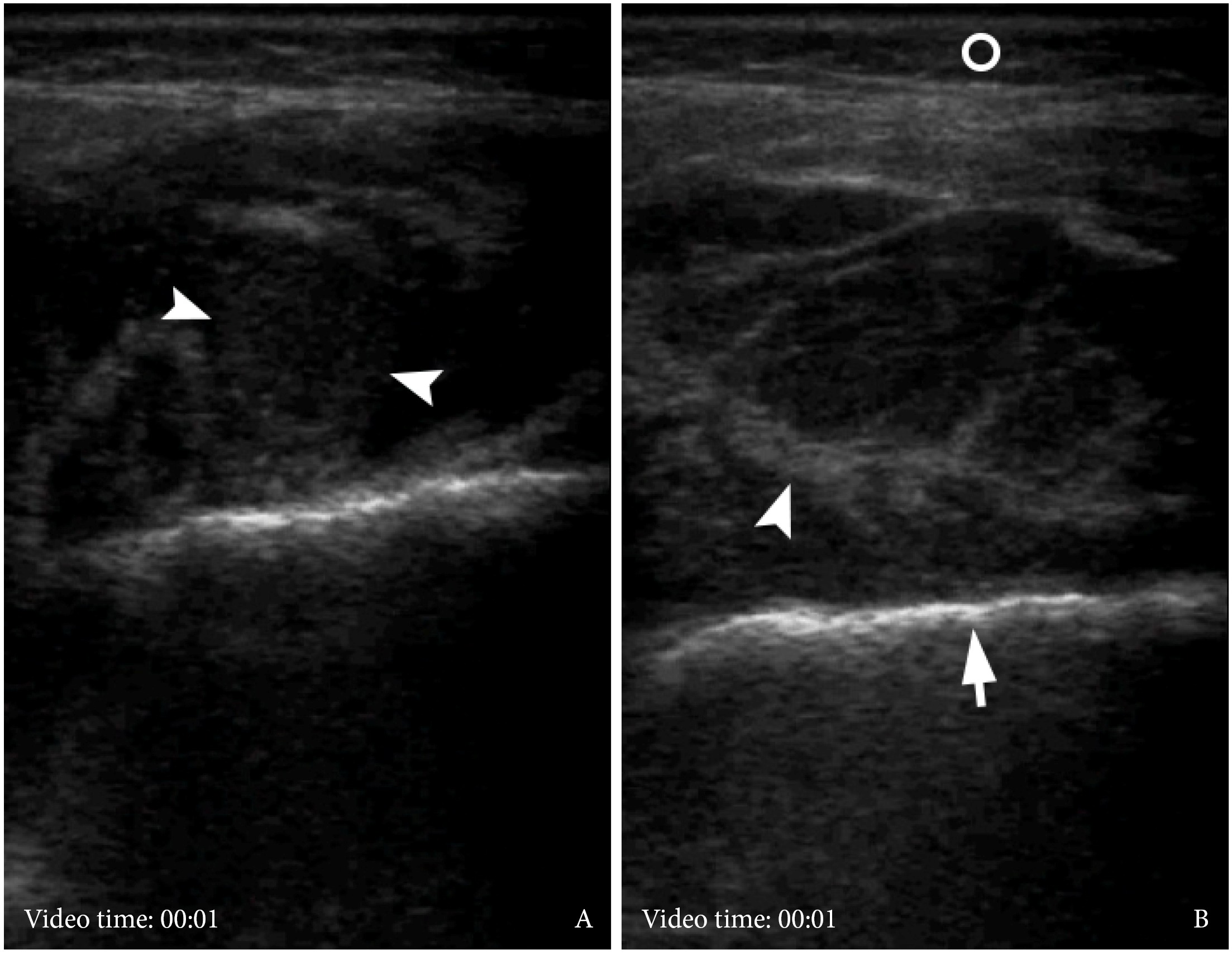
VIDEO 1. Supplemental Video Content shows the gray scale ultrasound examination of the right masseter muscle. A: Intramuscular collection of pus is indicated by arrowheads. B: A swelled subcutaneous adipose tissue is labeled by circle, blade tendon of the internal masseter – by arrowhead, outer cortical plate of ramus – by arrow. Video is available in the page of the full-text article on dtjournal.org and in the YouTube channel ‘Videos DTJournal’.
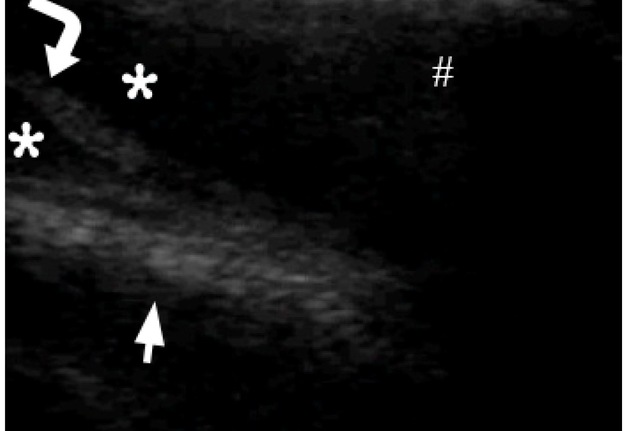
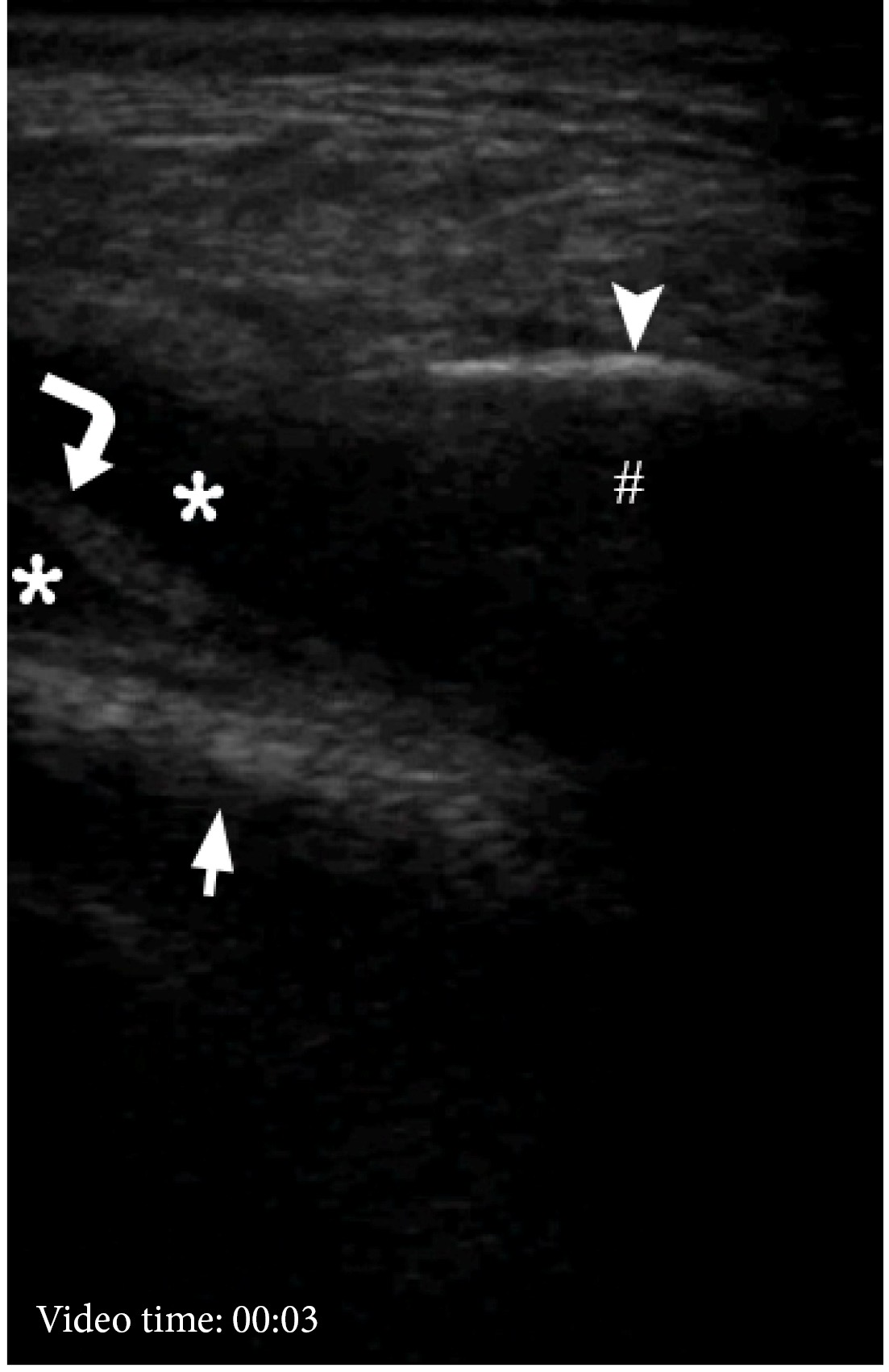
VIDEO 2. Supplemental Video Content shows the gray scale US examination of the lower part of the right temporal region. Arrowhead is indicated on outer surface of the right zygomatic arch, hashtag – on acoustic shadowing, asterisks – on pus, curved arrow – on fibers of temporalis, arrow – on surface of cranial bone. Video is available in the page of the full-text article on dtjournal.org and in the YouTube channel ‘Videos DTJournal’, available at Link
VIDEO 3. Supplemental Video Content demonstrates the gray scale ultrasound examination (A, B) of swelled soft tissues of the right parotid region. Epidermis and dermis are indicated by quadrate. Black arrow labeles temporoparietal fascia, white arrow – temporal fascia to the area of its split. Arrowheads indicate well-defined collection of hypoechoic heterogenic fluid (i.e., pus), which is located between periosteum (i.e., pericranium)and tendons/fibers of temporal muscle. Сurved arrow indicates suture between bones of the lateral side of the skull. Open circle labeles subcutaneous cellular tissue, closed circle – fat pad of temporal fascial, and waved arrow – lumen of the vessel located between temporoparietal fascia and temporal fascia. Video is available in the page of the full-text article on dtjournal.org and in the YouTube channel ‘Videos DTJournal’, available at Link
Fragiskos states that the term ‘cellulitis’ has prevailed over the term ‘phlegmon’ which was abandoned.15 He described cellulitis as an acute, diffuse inflammatory infiltration of the loose connective tissue found underneath the skin with no pus in initial stages and with purulent content in advanced stages.15 In other study have found that cellulitis (in some East European states it terms as inflammatory infiltrate) occurs in two forms: the first as an independent disease, the second as an early phase of a purulent inflammatory process.2 Bertolus et al by emphasizing that treatment only by antibiotics is possible only in early clinical stages of cellulitis, the advanced purulent stages can be complicated by necrotizing fasciitis, abscess, mediastinitis, thrombosis of the cavernous sinus, and septic shock.16, 17 Rath et al supported the previously mentioned authors and described cellulitis as a common, bacterial, non-purulent infection spreading diffusely along the skin. In their prospective study is also emphasized that suppurative form of cellulitis can also develop.18
All sources are unanimous in defining the term ‘abscess’ describing it as a limited collection of purulent content.
The authors from Post-Soviet countries predominantly terms the diffuse purulent inflammation in the soft tissues as ‘phlegmon.’2, 19 and are using it in the next combination: ‘phlegmon of floor of the mouth’, ‘phlegmon of the submandibular area’, ‘phlegmon of the neck’, etc. The authors from the rest of the world are widely using the term ‘phlegmon’ in head neck, thoracic, abdominal surgery, and orthopaedics in the next combinations: ‘retropharyngeal phlegmon20, 21’, ‘phlegmonous esophagogastritis22’, ‘abdominal phlegmon23’, ‘appendicial phlegmon24’, ‘phlegmon of the hand25, 26’, ‘phlegmon of the digital flexor tendon sheaths27’, etc.
The term ‘necrotizing fasciitis (NF) has synonyms haemolytic streptococcus gangrene, and flesh-eating bacteria syndrome.28 NF of the head and neck is usually named as cervicofacial necrotizing fasciitis17 and described according to Marchesi et al as an infection which proceeds from the subcutaneous cellular tissue to the underlying superficial and deep fascial planes causing tissue necrosis that does not involve muscles.
Malik et al29 and Chunduri et al30 stated that NF may also be accompanied by necrosis of muscles, gland tissue and even bone. Puvanendran et al31 indicated classification of NF according to:
-
Microbiology (polymicrobial or monomicrobial).
-
Anatomy.
-
Depth of infection.
Another term, ‘purulent-necrotic phlegmon’, which is frequently uses by authors from East European states, described as purulent conditions of the soft tissues which associated with its necrosis and presence of tissues which looked as a ‘boiled meat2.’ And to our opinion it can be considered as synonymous with the NF.
Understanding the anatomical landmarks and ultrasound appearance of the parotid masseter and temporal region at non-symptomatic and swelled sides is also crucial in understanding and describing of our case.
Fascial coverage of the masseter muscle and parotid gland is perfectly described by Hînganu et al.32 Masseteric and parotid fascias (also used a collective term for describing both fascias – ‘parotideomasseteric fascia’2, 33 [synonym: parotideomasseteric fascia9, parotid-masseteric fascia34]) present distinctive structures on ultrasound images. During US they are visualized as very thin hyperechoic linear structure which covers lateral surface of the masseter muscle and parotid. Tymofieiev stated that parotid fascia, which forms a capsule for parotid gland, gives many processes inside the gland, which in the form of processes divide it into separate lobules.2
Masseter muscle consists of three sections: 1) superficial, 2) middle, and 3) deep (internal masseter).11 Busato et al11 described that the deep masseter composed of 3 layers: outward, middle, and inward.
Analyzing ultrasound images of the temporal area, we adhered to the standardized nomenclature of Davidge et al35 for the anatomic structures of the temporoparietal region:
-
Skin.
-
Subcutaneous adipose tissue.
-
Temporoparietal fascia.
-
Loose areolar tissue plane.
-
Superficial leaflet of temporal fascia.
-
Fat pad of temporal fascia.
-
Deep leaflet of temporal fascia
-
Fat pad deep to temporal fascia.
-
Temporalis (synonym: temporal muscle).
-
Pericranium (i.e., periosteum of the outer side of the cranial bones).
It should be noted, that the presence or absence of fat pad between temporoparietal fascia and superficial leaflet of temporal fascia may be variable and depend on individual characteristics, such as obesity.35 Markiewicz, Ord, and Fernandes stated that temporoparietal fascia is continuous below the zygomatic arch as the superficial musculo-aponeurotic system (SMAS).36
Many fibers of temporalis muscle originate from the inner side of this temporalis fascia, making it difficult not only to detach the last from the muscular belly but also to distinguish deep leaflet of the fascia during US.33
Mallorie et al clearly proved, based on 43 cases, that sensitivity and specificity of US in identification of purulent content (in head and neck infection cases) were very high, 96% and 82%, respectively.1 On US images the pus is visualized as homogenous/heterogenous hypoechogenic content.8 In some cases fluctuating of the collected pus can be visualized upon applying a pressure by transducer. Hwang and Adler and Toprak et al described US appearance of 1) cellulitis – subcutaneous edema and subcutaneous fatty tissue appears thickened and echogenic, 2) abscess – frequently seen as irregular‑walled, septated, complex cystic lesion containing fluid with debris or internal echoes inside.37, 38 US‑guided aspiration is very useful technique in case of doubts in a surgical team.38 Color and power Doppler can usually show a prominent flow in the area of inflammation.37 Understanding the US landscape of zygomatic arch39 area and anatomic structures located medially to it is also important in realizing all aspects of our masticator space infection.
So, the authors from East European countries usually term the diffuse purulent inflammation without tissues necrosis in the region of masseter muscle as “phlegmon of parotid masseter region” with branching of the diagnosis, depending on the depth of purulent content localization, as “phlegmon of superficial/deep layers of the parotid masseter region.”2 Other authors name the purulent conditions in that area and/or temporal region as “masticator space infection.”3, 5 Masticator space40 according to literature is composed of a suprazygomatic and infrazygomatic portion.3 And the infrazygomatic portion is also separated by the mandibular ramus into medial and lateral (contains the masseter muscle) parts.3 Taking into account terminological data which we analyzed above, US images obtained before surgery, clinical and intraoperative appearance, and post-operative every day follow-up during 1 week after operation we stick to the pre-operative diagnosis (odontogenic phlegmon of the deep layers of right parotid masseter and temporal region from the tooth 4.7). In our case, we did not use the term ‘purulent form/stage of facial cellulitis’ to avoid any ambiguity (with its non-purulent forms). The term ‘phlegmon’ 1) has a clear difference essentially from the terms ‘abscess,’ ‘necrotizing fasciitis,’ 2) avoid any ambiguity with different stages of cellulitis, and 3) short enough not to overload diagnosis. But we agree that changing the words in the diagnosis to ‘odontogenic phlegmon of the right masticator space from the tooth 4.7’ will make the diagnosis easier despite the fact that anatomically the word ‘space’ does not accurately describe anatomical structures in that area and maybe will be better to be replaced with word ‘region.’
To our knowledge, there is no article highlighting US appearance of odontogenic phlegmon of parotid masseter and temporal regions, presenting both, images and videos. Intraoperative data of our report have clearly confirmed the information from preoperative sonograms in which layers the pus was located. Thus, head neck ultrasound can provide a real help in identifying pus collections and it’s spreading in case of purulent processes in deep-tissues of temporal and masseter regions as parts of masticator space.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ROLE OF THE AUTHORS
The authors are equally contributed to that paper.
FUNDINGS
No funding was received for this study.
ACKNOWLEDGMENTS
None.
REFERENCES (40)
-
Mallorie CN, Jones SD, Drage NA, Shepherd J. The reliability of high resolution ultrasound in the identification of pus collections in head and neck swellings. Int J Oral Maxillofac Surg 2012;41:252–5. Crossref
-
Tymofieiev OO. Manual of maxillofacial and oral surgery [Russian]. 5th ed. Kyiv: Chervona Ruta-Turs; 2012.
-
Ko IC, Yoon KH, Park KS, Cheong, JK, Bae JH, Lee KW, Chin YJ. An unusual abscess formation in the masticator space after acupressure massage: a case report. J Korean Assoc Oral Maxillofac Surg 2015;41:52–6.Crossref
-
de Oliveira Neto PJ, de Souza Maliska MC, Sawazaki R, Asprino L, de Moraes M, Moreira RW. Temporal abscess after third molar extraction in the mandible. Oral Maxillofac Surg 2012;16:107–10. Crossref
-
Shin J, Park SI, Cho JT, Jung SN, Byeon J, Seo BF. Necrotizing fasciitis of the masticator space with osteomyelitis of the mandible in an edentulous patient. Arch Craniofac Surg 2019;20:270–3. Crossref
-
Baurmash HD. Ultrasonography in the diagnosis and treatment of facial abscesses. J Oral Maxillofac Surg 1999;57:635–6. Crossref
-
Pandey PK, Umarani M, Kotrashetti S, Baliga S. Evaluation of ultrasonography as a diagnostic tool in maxillofacial space infections. J Oral Maxillofac Res 2012;2:e4. Crossref
-
Chang KP, Chen YL, Hao SP, Chen SM. Ultrasound-guided closed drainage for abscesses of the head and neck. Otolaryngol Head Neck Surg 2005;132:119–24. Crossref
-
Al-Belasy FA. Ultrasound-guided drainage of submasseteric space abscesses. J Oral Maxillofac Surg 2005;63:36–41. Crossref
-
Gudi SS, Sarvadnya J, Hallur N, Sikkerimath BC. Ultrasound guided drainage of submasseteric space abscesses. Ann Maxillofac Surg 2013;3:31–4. Crossref
-
Busato A, Balconi G, Vismara V, Bertelè L, Garo G, DE Gregorio D. Ultrasound and analysis of the deformation patterns of the masseter muscle: comparing surgical anatomy, ultrasound and functional anatomy. Oral Implantol (Rome) 2017;9(Suppl 1/2016 to N 4/2016):28–37. Crossref
-
Liao L-J, Lo W-C. High-resolution sonographic measurement of normal temporomandibular joint and masseter muscle. J Med Ultrasound 2012;20:96–100. Crossref
-
Fernandes T, Lobo JC, Castro R, Oliveira MI, Som PM. Anatomy and pathology of the masticator space. Insights Imaging 2013;4:605-16. Crossref
-
Flynn TR. Principles of management of odontogenic infections. In: Miloro M, Ghali GE, Larsen PE, Waite PD, editors. Peterson`s principles of oral and maxillofacial surgery, 2nd ed. BC Decker Inc, 2004:277–94.
-
Fragiskos FD. Odontogenic infections. In: Fragiskos FD, editor. Oral surgery, 1st ed. Springer-Verlag, 2007:205–42.
-
Bertolus C, Schouman T, Aubry A, Hausfater P. Is procalcitonin a useful biomarker for the risk stratification of facial cellulitis? J Craniomaxillofac Surg 2016;44:995–7. Crossref
-
Lee JW, Immerman SB, Morris LG. Techniques for early diagnosis and management of cervicofacial necrotising fasciitis. J Laryngol Otol 2010;124:759–64.
-
Rath E, Skrede S, Mylvaganam H, Bruun T. Aetiology and clinical features of facial cellulitis: a prospective study. Infect Dis (Lond) 2018;50:27–34. Crossref
-
Durnovo EA, Furman IV, Pushkin SY, Maslennikov IA, Bondar OG, Ivanitsky GR. Clinical results of the application of perftoran for the treatment of odontogenous abcesses and phlegmons in the maxillofacial region. J Craniomaxillofac Surg 2008;36:161–72. Crossref
-
Carter JM, Patel A, Evans SS, Lakey M, Rodriguez KH. Retropharyngeal phlegmon in Rosai Dorfman disease. Int J Pediatr Otorhinolaryngol 2014;78:373–6. Crossref
-
Duval M, Daniel SJ. Retropharyngeal and parapharyngeal abscesses or phlegmons in children. Is there an association with adenotonsillectomy? Int J Pediatr Otorhinolaryngol 2008;72:1765–9. Crossref
-
Kim HS, Hwang JH, Hong SS, Chang WH, Kim HJ, Chang YW, Kwon KH, Choi DL. Acute diffuse phlegmonous esophagogastritis: a case report. J Korean Med Sci 2010;25:1532–5. Crossref
-
Jeyalingam T, O’Donnell S, Chawla T, Erin K, Nguyen GC, Silverberg MS, Steinhart A, Croitoru K. Anti-TNF vs surgical management of abdominal phlegmon in Crohn`s disease: a retrospective analysis. J Can Assoc Gastroenterol 2018;1:191–2. Crossref
-
Zhang H, Bai Y, Wang W. Nonoperative management of appendiceal phlegmon or abscess in children less than 3 years of age. World J Emerg Surg 2018;13:10. Crossref
-
Mamane W, Lippmann S, Israel D, Ramdhian-Wihlma R, Temamb M , Masb V, Pierrartc J, Emmanuel H. Masmejean EH. Infectious flexor hand tenosynovitis: state of knowledge. A study of 120 cases. J Orthop 2018;15:701–6. Crossref
-
Boyer E, Igeta, Y, Facca, S, Xavier F, Liverneaux, P, Prunières, G. Surgical treatment of phlegmons of the digital flexor tendon sheaths at the early stage: lavage by conventional open technique versus ultrasound-guided percutaneous technique. Ann Chir Plast Esth 2019;64:344–50. Crossref
-
Montechiarello S, Miozzi F, Martinelli M, Giovagnorio F. Ultrasound picture of a wooden splinter evolved in phlegmon of the hand. J Ultrasound 2010;13:38–40. Crossref
-
Marchesi A, Marcelli S, Parodi PC, Perrotta RE, Riccio M, Vaienti L. Necrotizing fasciitis in aesthetic surgery: a review of the literature. Aesthetic Plast Surg 2017;41:352–8. Crossref
-
Malik V, Gadepalli C, Agrawal S, Inkster C, Lobo C. An algorithm for early diagnosis of cervicofacial necrotising fasciitis. Eur Arch Otorhinolaryngol 2010;267:1169–77. Crossref
-
Chunduri NS, Madasu K, Tammannavar PS, Pushpalatha C. Necrotising fasciitis of odontogenic origin. BMJ Case Rep 2013;2013:bcr2012008506. Crossref
-
Puvanendran R, Huey JC, Pasupathy S. Necrotizing fasciitis. Can Fam Physician 2009;55:981–7.
-
Hînganu D, Stan CI, Ciupilan C, Hînganu MV. Anatomical considerations on the masseteric fascia and superficial muscular aponeurotic system. Rom J Morphol Embryol 2018;59:513–6.
-
Stecco C, Hammer W, Vleeming A, De Caro R. Fasciae of the head and neck. In: Stecco C (author), Hammer W (English editor). Functional atlas of the human fascial system, 1st ed. Churchill Livingstone (Elsevier), 2015:103–39. Crossref.
-
Sati S, Makki AS, Shashikant M, Ramasastry SS. Scalp reconstruction. In: Weinzweig J, editor. Plastic surgery secrets plus. 2nd ed. Mosby (Elsevier), 2010:422–6. Crossref
-
Davidge KM, van Furth WR, Agur A, Cusimano M. Naming the soft tissue layers of the temporoparietal region: unifying anatomic terminology across surgical disciplines. Neurosurgery 2010;67(3 Suppl Operative):ons120–9; discussion ons129–30. Crossref
-
Markiewicz MR, Ord R, Fernandes RP. Local and regional flap reconstruction of maxillofacial defects. In: Brennan PA, Schliephake H, Ghali GE, Cascarini L, editors. Maxillofacial surgery, 3rd ed. Churchill Livingstone (Elsevier), 2017:616–35. Crossref
-
Hwang S, Adler RS. Sonographic evaluation of the musculoskeletal soft tissue masses. Ultrasound Q 2005;21:259–70.
-
Toprak H, Kiliç E, Serter A, Kocakoç E, Ozgocmen S. Ultrasound and Doppler US in evaluation of superficial soft-tissue lesions. J Clin Imaging Sci 2014;4:12. Crossref
-
Buller J, Zirk M, Kreppel M, Maus V, Zöller JE. Intraoperative ultrasound control of zygomatic arch fractures: does additional imaging improve reduction quality? J Oral Maxillofac Surg 2019;77:769–76. Crossref
-
Evans R. Anatomy and technique. In: Ahuja AT, Evans R, editors. Practical head and neck ultrasound, 1st ed. Greenwich Medical Media Limited, 2000:1–17.