Features of Diagnostics, Clinical Course and Treatment of the Branchial Cleft Cysts
February 18, 2017
https://doi.org/10.23999/j.dtomp.2017.1.3
J Diagn Treat Oral Maxillofac Pathol 2017;1:15–31.
Under a Creative Commons license
How to cite this article
Tymofieiev OO, Fesenko II, Cherniak OS, Zaritska VI. Features of diagnostics, clinical course and treatment of the branchial cleft cysts. J Diagn Treat Oral Maxillofac Pathol 2017;1(1):15–31.
INSTITUTIONAL REPOSITORY
https://ir.kmu.edu.ua/handle/123456789/785
Contents: Abstract | Introduction | Clinical Picture | Pathology | Ultrasound | CT & MRI | Treatment | Complications | Prognosis | References (35)
Abstract
Purpose: The aim of the present study was to determine the features of diagnostics, clinical course and treatment of the branchial cleft cysts.
Patients and Methods: The study composed of the branchial cleft cysts investigation and their complications in patients of different age groups, methods of diagnostics, anatomical features, surgical stages and pathomorphological study.
Results: Diagnostic value of sonography, MDCT and MRI, pathomorphological study in verification of branchial cleft cysts and their complications have been proved. Surgical treatment technique is presented.
Conclusion: Presented methods of diagnostics of the branchial cleft cysts and their complications, variants of clinical course and treatment can reduce the risk of failure at the pre-, intra- and post-operative stages.
Introduction
Branchial cleft cyst (synonyms: lateral cyst of the neck, congenital lateral cyst of the neck, branchial cyst, lateral branchial cyst of the neck, lateral lymphoepithelial cyst) according to our data, found in 25% among all cysts of the soft tissue in maxillofacial and neck area [1-15]. The branchial cleft fistulas are rarely detected.
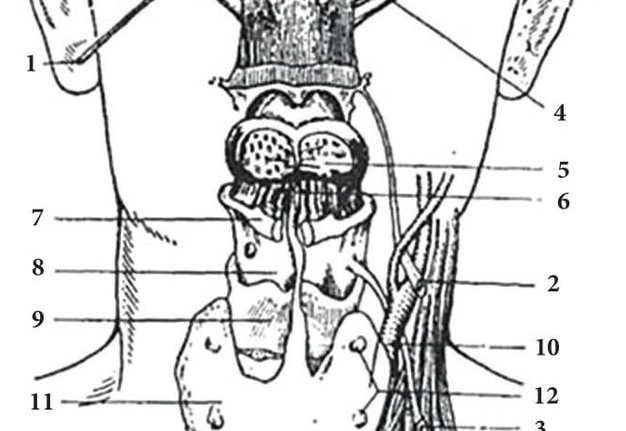
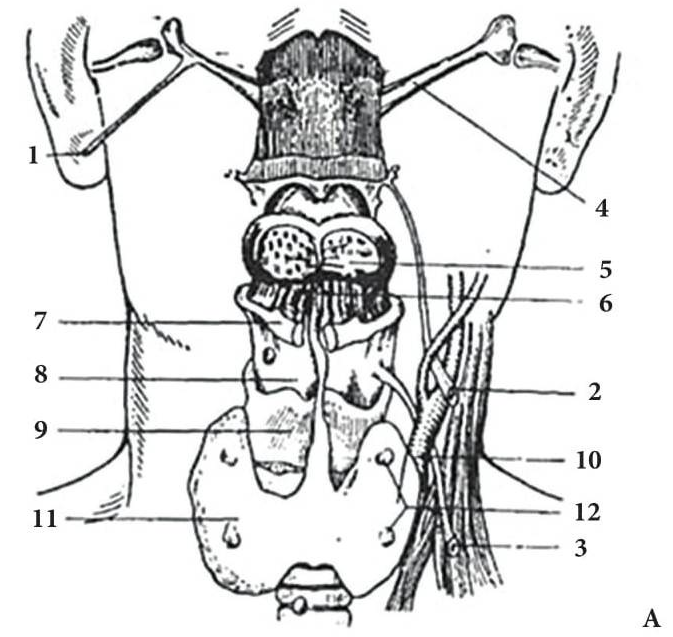
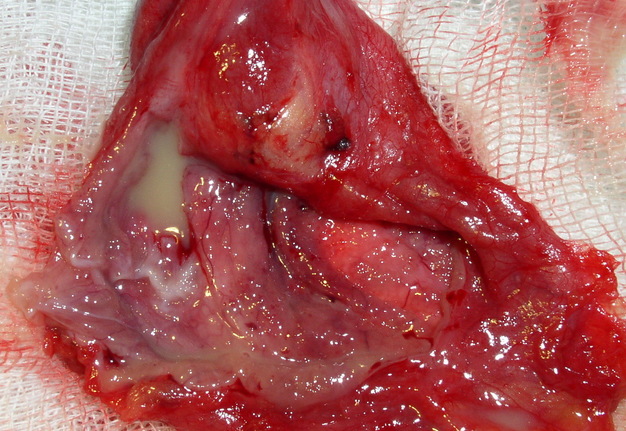
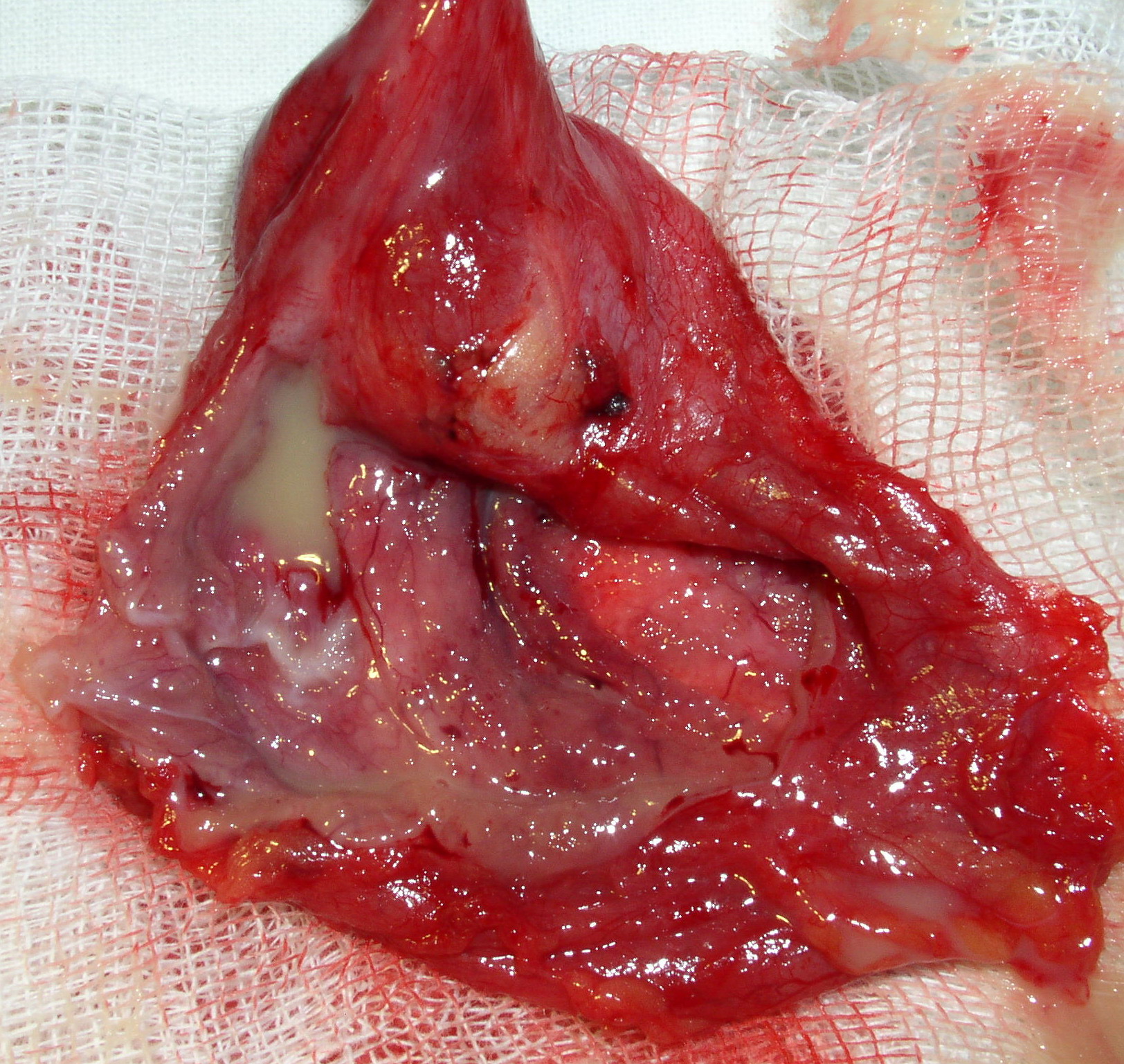
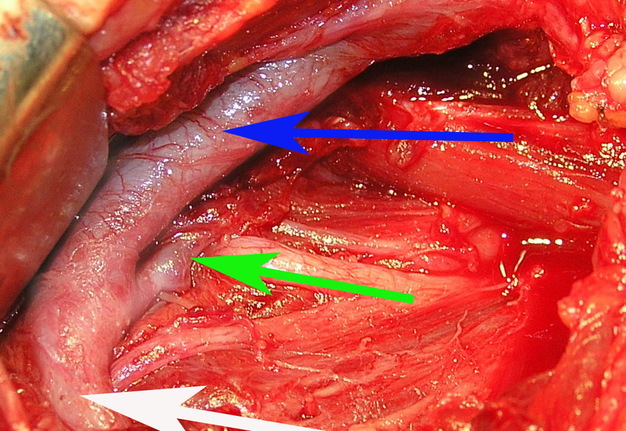
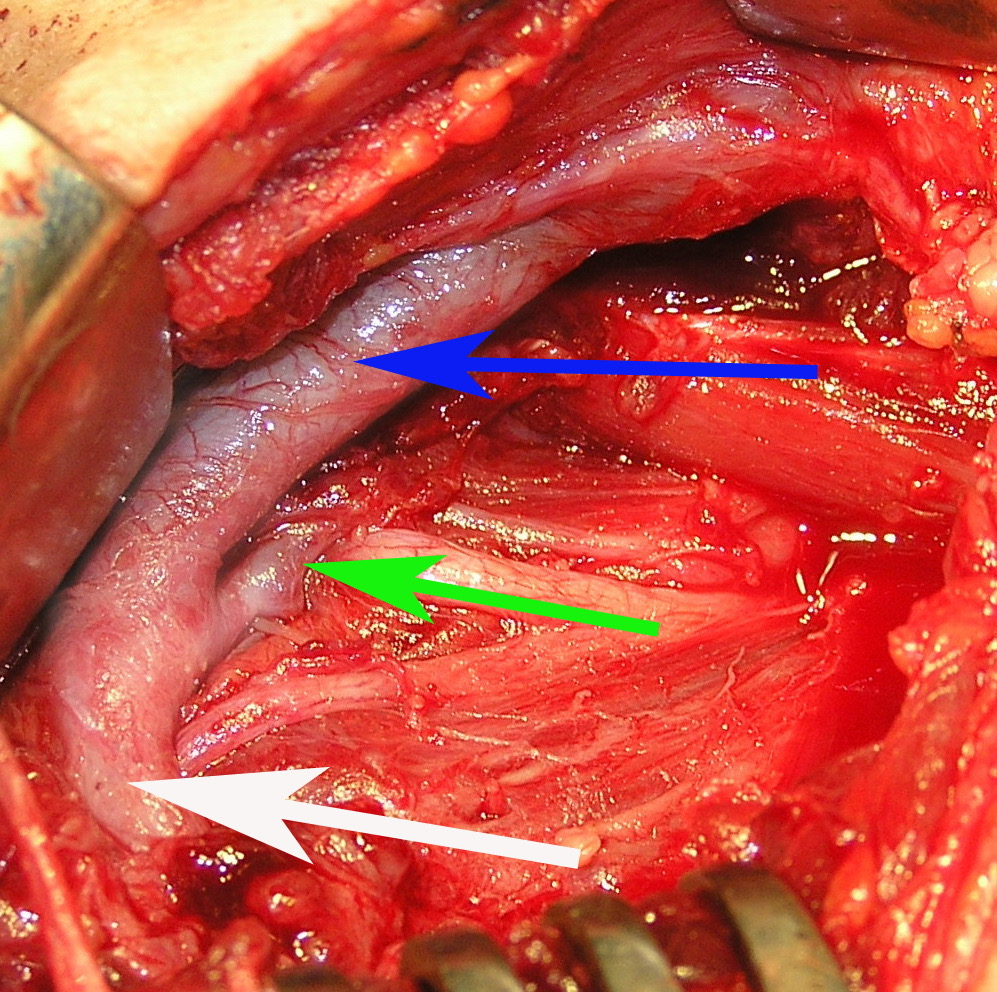
FIGURE 1A. Location scheme of branchial neck fistulas (A): 1 – I branchial pocket; 2 – II branchial pocket; 3 – III branchial pocket; 4 – auditory tube; 5 – tongue; 6 – thyroglossal duct; 7 – the hyoid bone; 8 – thyroid-hyoid membrane; 9 – thyroid cartilage; 10 – common carotid artery; 11 – thyroid gland; 12 – parathyroid glands. Location of the inner pole (B) of BCC after its removal. Internal jugular vein is marked by white arrow, facial vein – yellow arrow, posterior belly of the digastric muscle – green arrow.
FIGURE 1B. Location scheme of branchial neck fistulas (A): 1 – I branchial pocket; 2 – II branchial pocket; 3 – III branchial pocket; 4 – auditory tube; 5 – tongue; 6 – thyroglossal duct; 7 – the hyoid bone; 8 – thyroid-hyoid membrane; 9 – thyroid cartilage; 10 – common carotid artery; 11 – thyroid gland; 12 – parathyroid glands. Location of the inner pole (B) of BCC after its removal. Internal jugular vein is marked by white arrow, facial vein – yellow arrow, posterior belly of the digastric muscle – green arrow.
Branchial cleft cysts (Greek, branchia gill) have dysontogenetic origin.
With regard to the pathogenesis of branchial cleft cysts (BCCs) and fistulas there is disagreement till present day. There are two theories of its origin. According to the "thymus" theory these cysts and fistulas are formed from the remnants of thymopharyngeal duct. "Branchial" theory links the origin of these lesions with abnormal development of branchial (pharyngeal) pockets. Anomalies of the 2nd or 3rd pair of pharyngeal (branchial) pockets are the source of the formation of the BCCs and fistulas. Internal branchial pockets formed by endoderm and the external (or grooves) by ectodermal germ layers. BCCs can be both endodermal and ectodermal origin (Fig 1A).
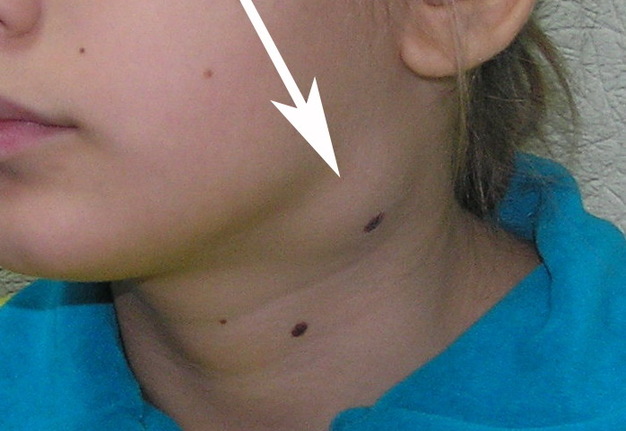
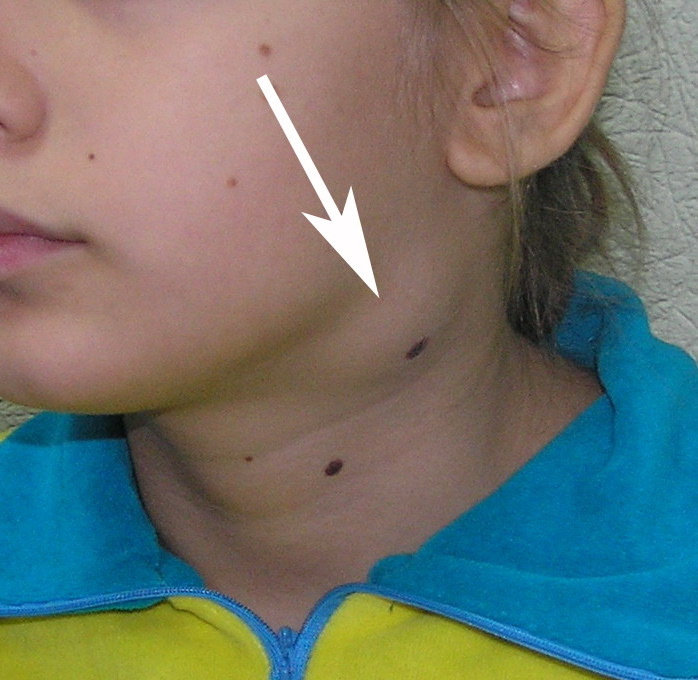
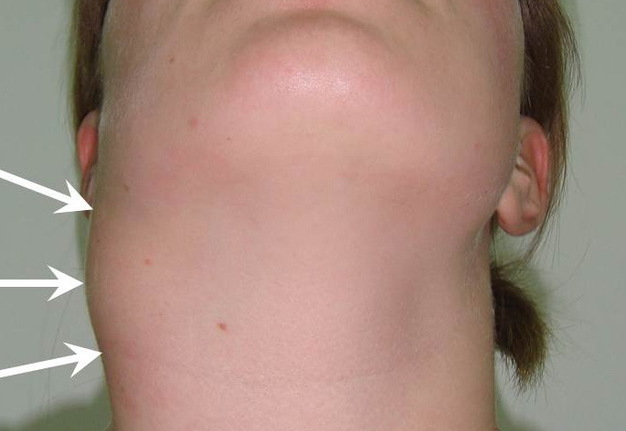
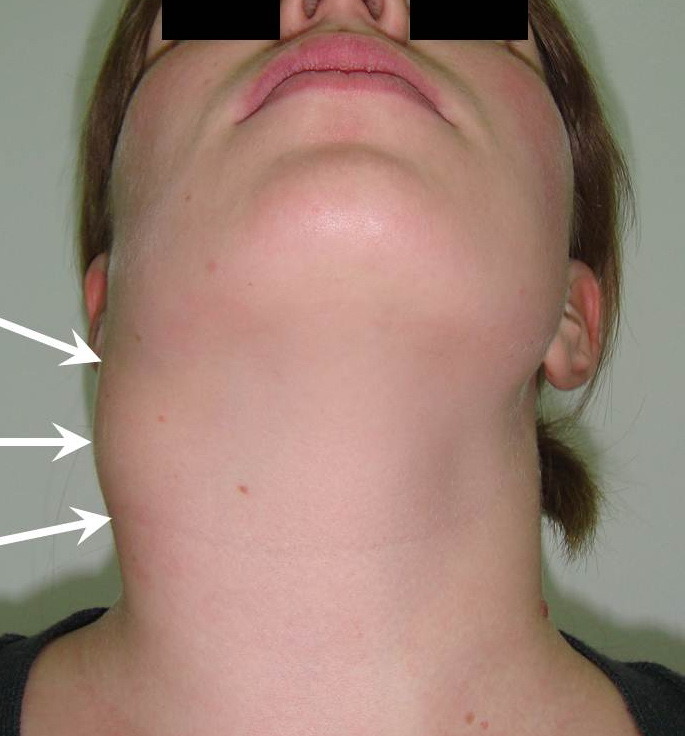
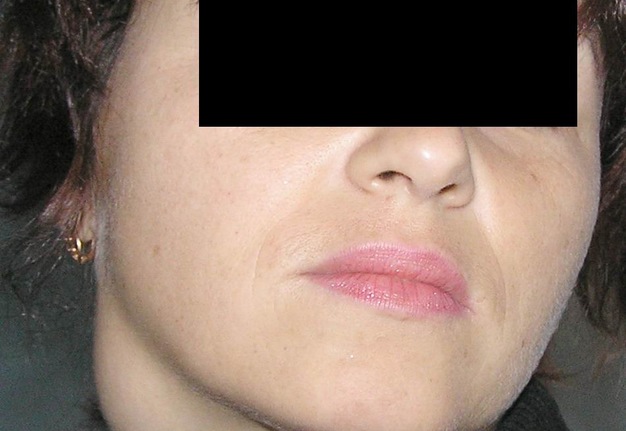
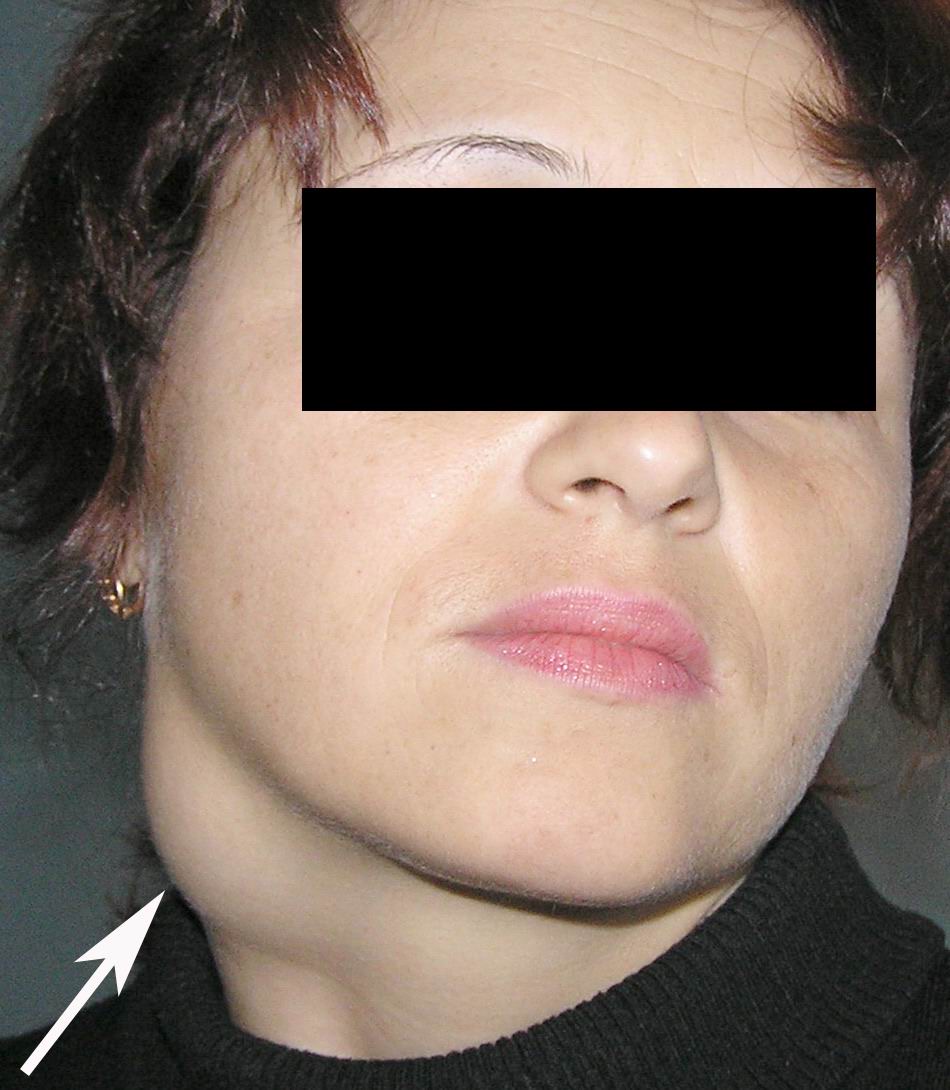
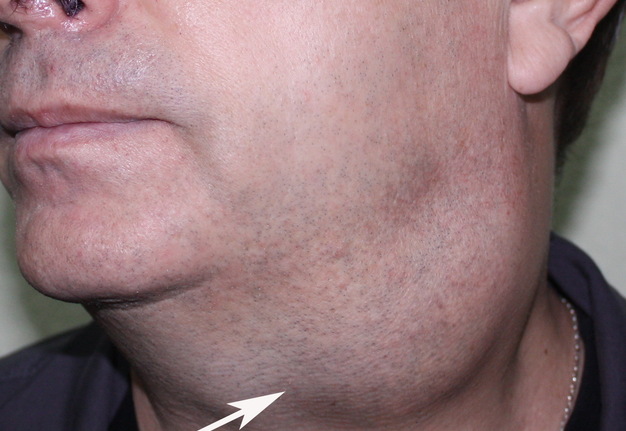
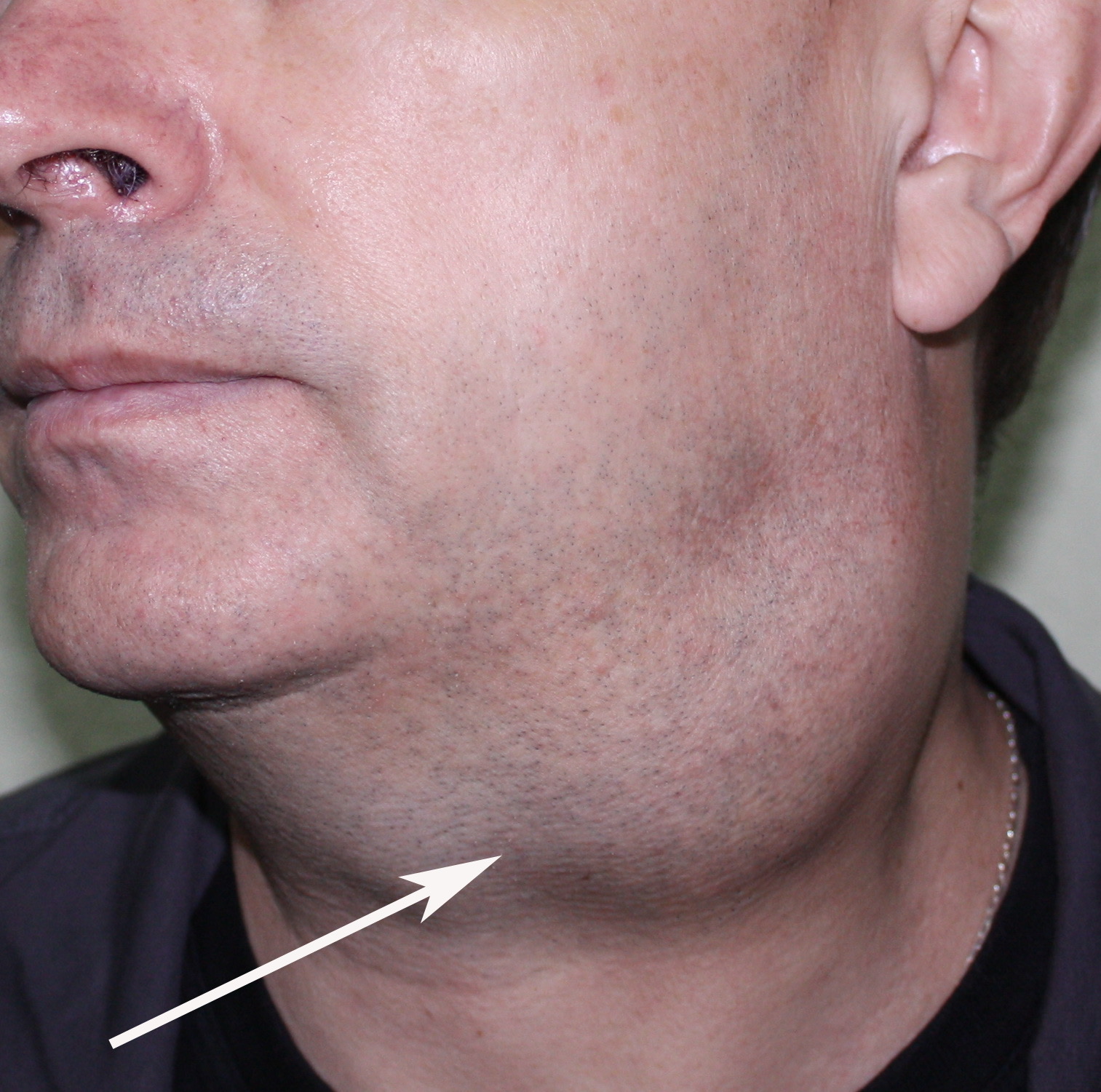
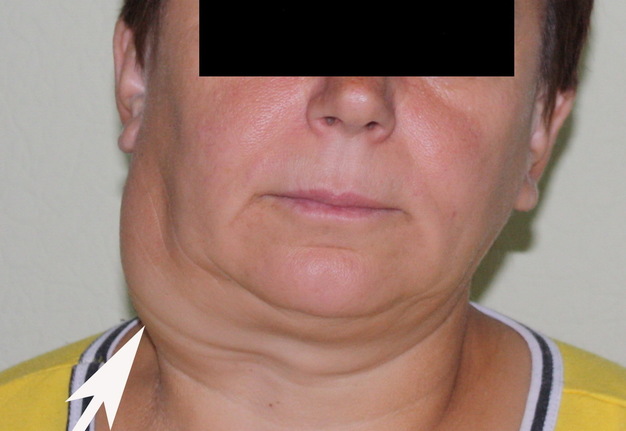
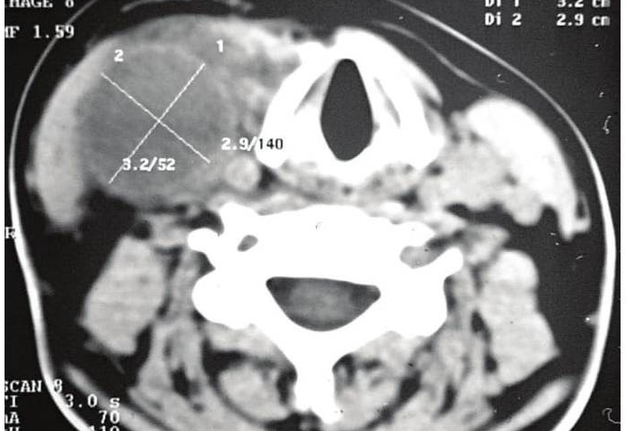
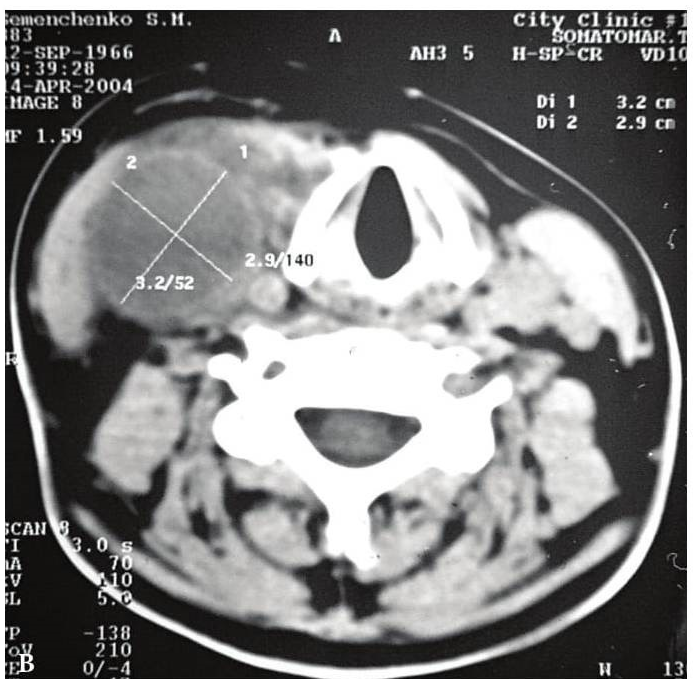
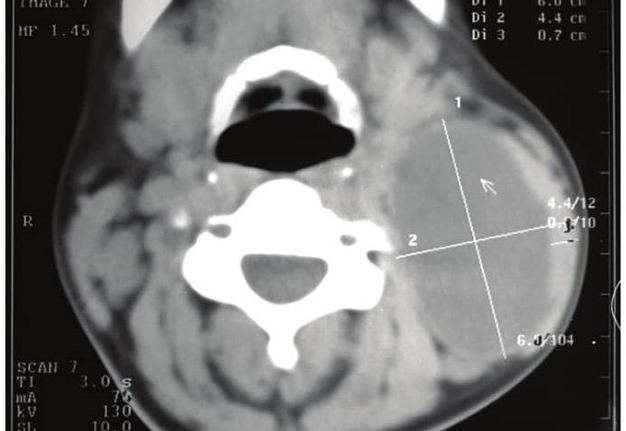
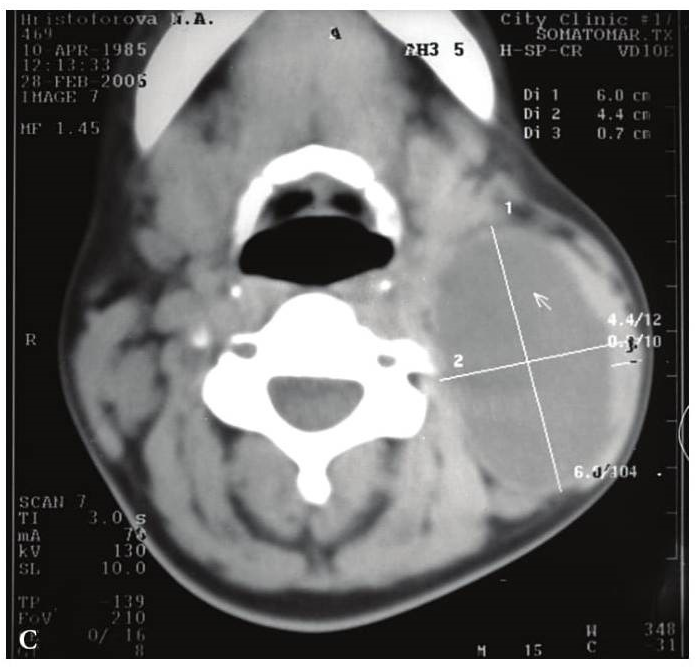
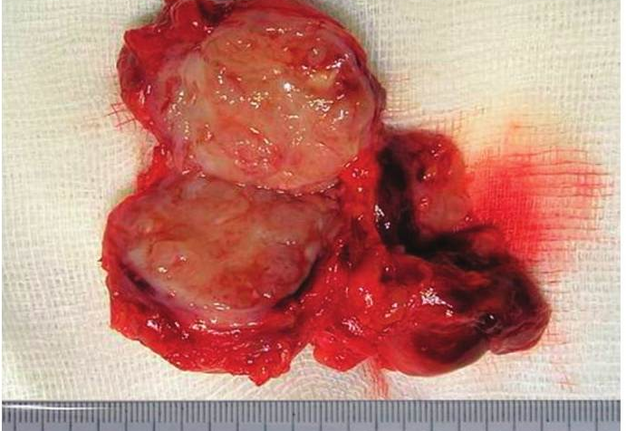
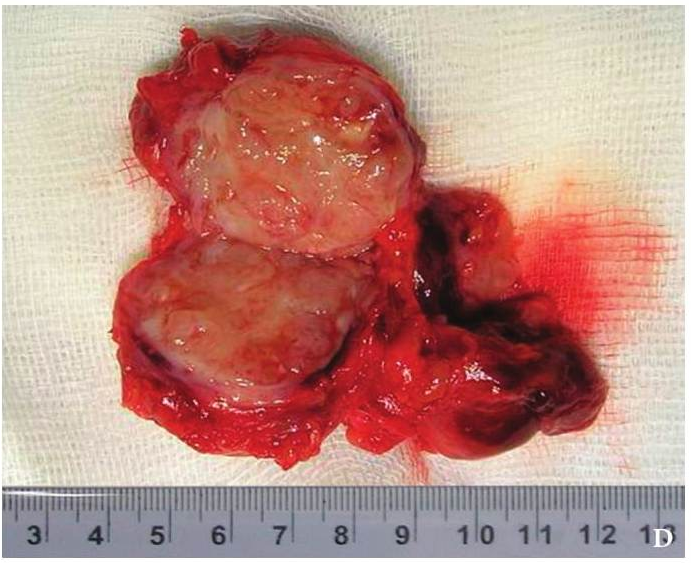
Cysts occur at any age, but are much more common in children and young adults (Fig 2). Their appearance is preceded (provoke) by the infections of the respiratory tracts (tonsillitis, flu, etc.). The sizes of the BCCs can be different (Fig 2). In contrary to dermoid (epidermoid) cysts the BCCs are often suppurate [1-3].
First, BCCs were classified according to their localization. Bailey H. (1929), divided them into 4 types [16]: type 1 – deep to platysma, anterior to sternocleidomastoid (SCM); type 2 – abutting internal carotid artery and adherent to internal jugular vein (most common); type 3 – extending between internal and external carotid arteries; type 4 – abutting pharyngeal wall and potentially extending superiorly to skull base.
CLINICAL PICTURE
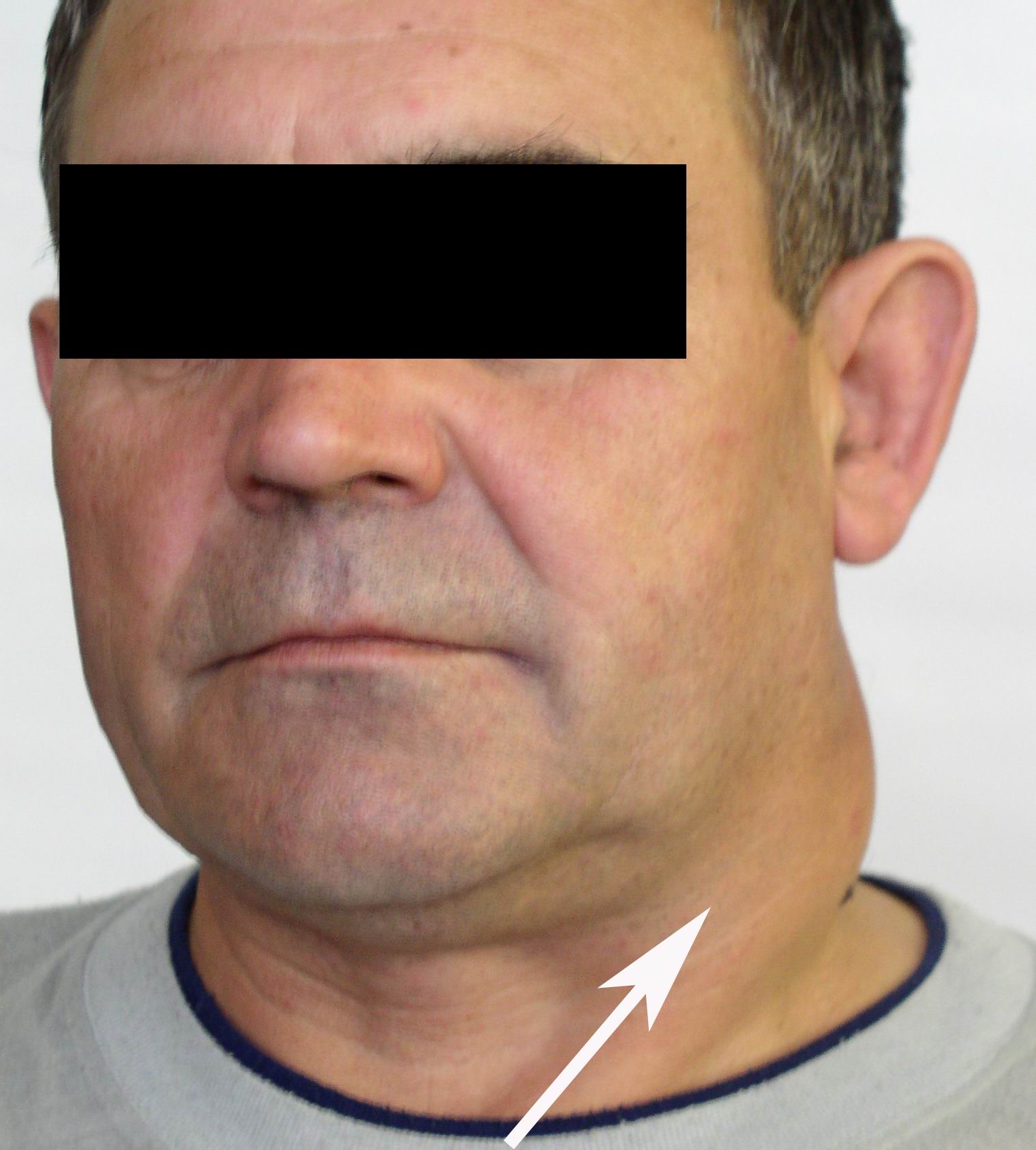
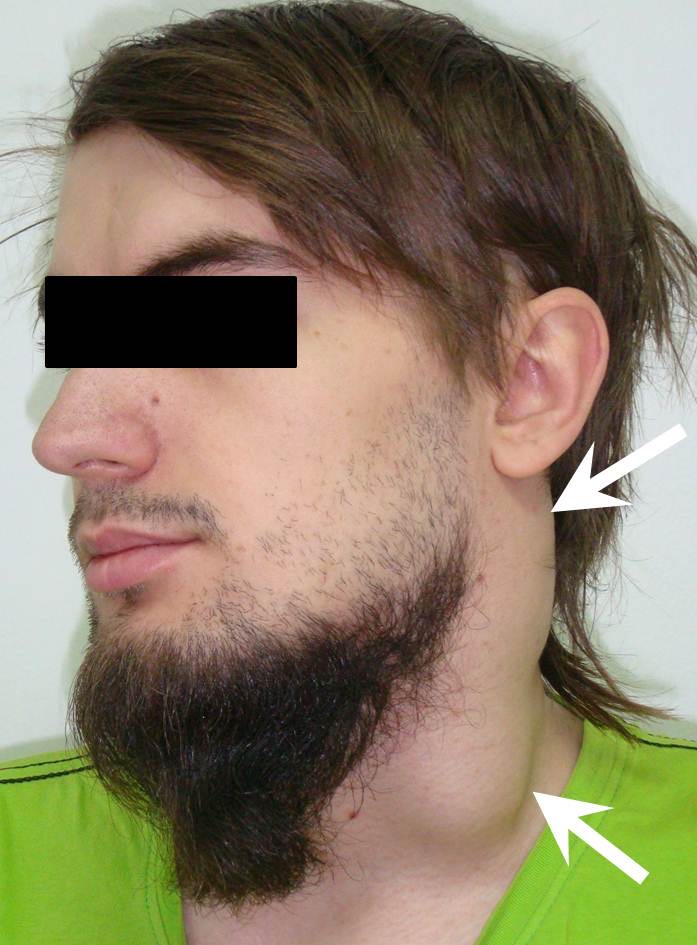
BCCs are the round-shaped mass at the upper neck anteriorly to the sternocleidomastoid muscle (in carotid triangle). While it may located in the middle and even lower parts of the neck. Typically, the lateral cyst localized in the upper or middle third of the neck adjacent to the anterior edge of the sternocleidomastoid muscle or partly comes under it. It is located between the 2nd and 3rd fascial leaf of the neck (between the superficial and deep fascia leaf of the own neck fascia) on the neurovascular bundle. The upper pole of the cyst is often found near or under the posterior edge of the digastric or stylohyoid muscles. Medially the cysts are adjacent to the internal jugular vein at the level of common carotid artery bifurcation. BCCs can be located in the upper, middle and lower parts of the neck. Along the length of the cyst may extend down to the clavicle, and in the upper part of the neck reaches mastoid process (Fig 1B).
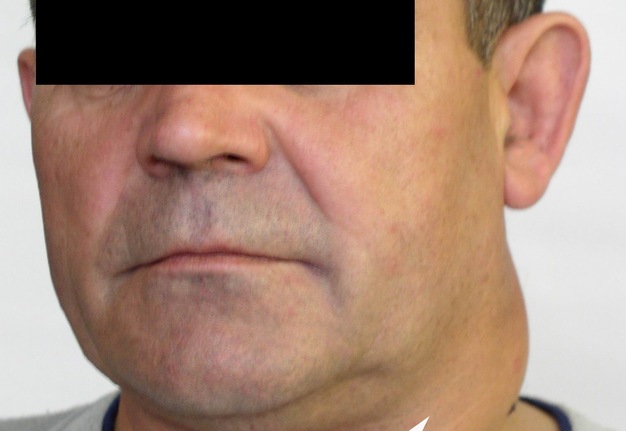
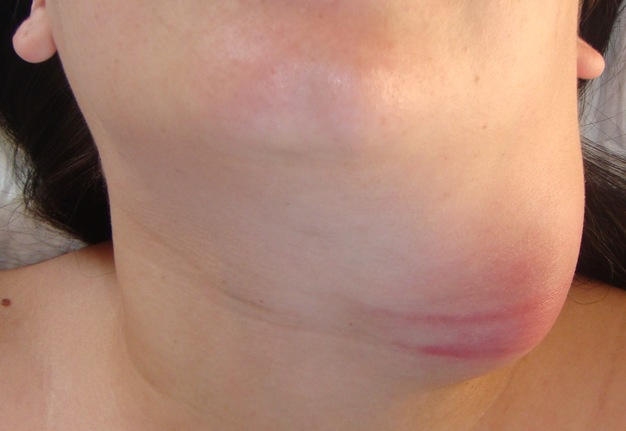
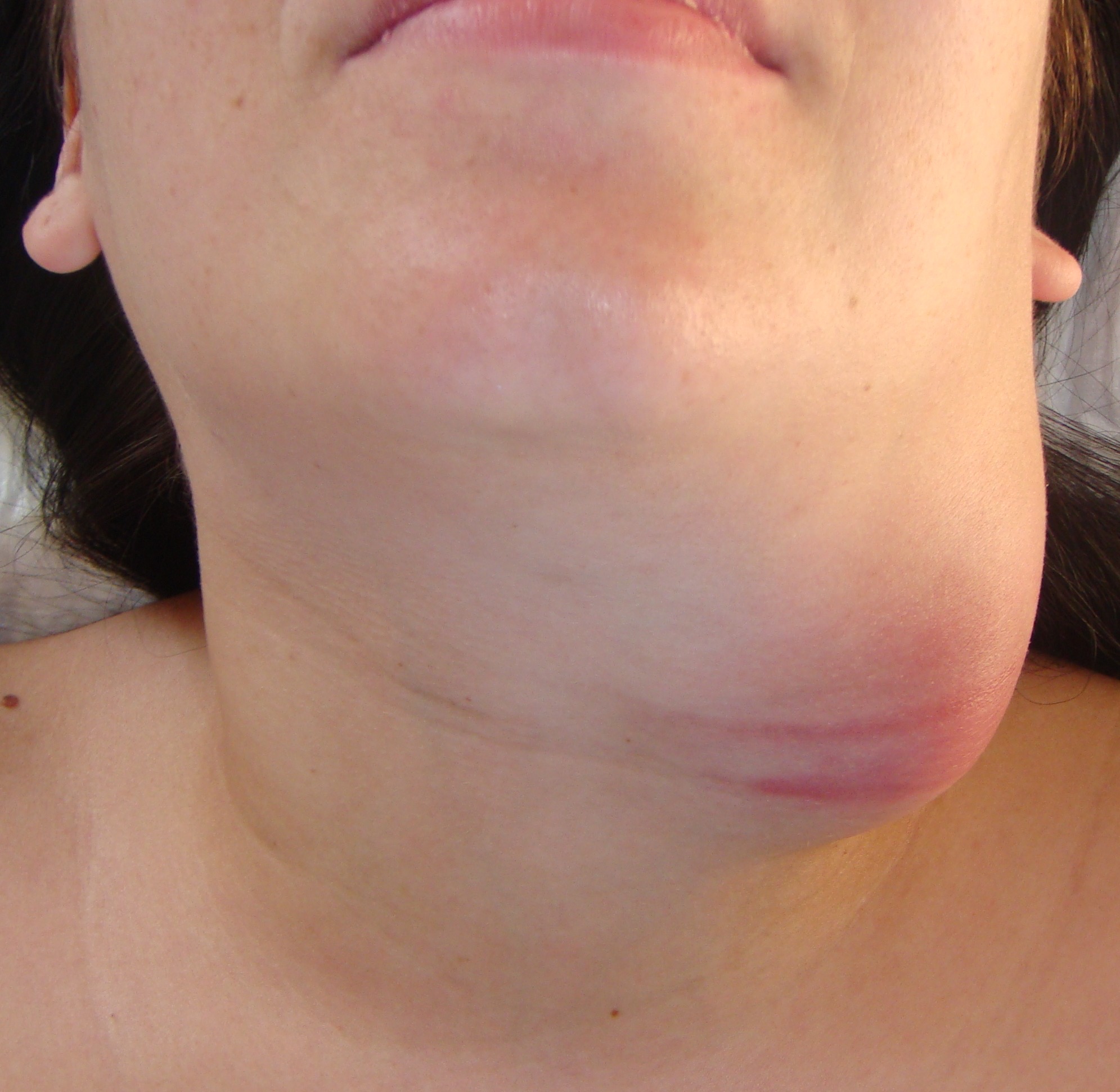
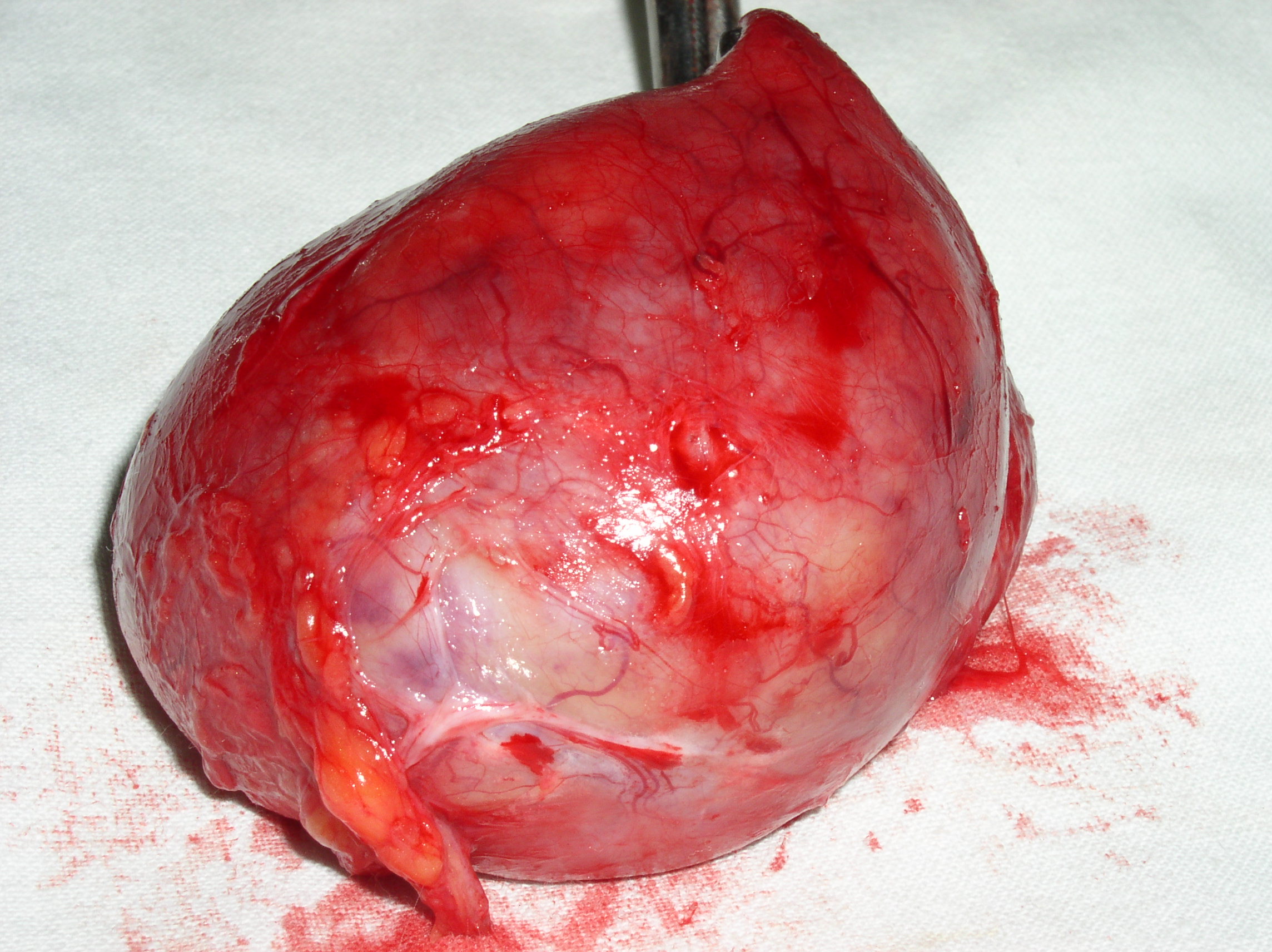
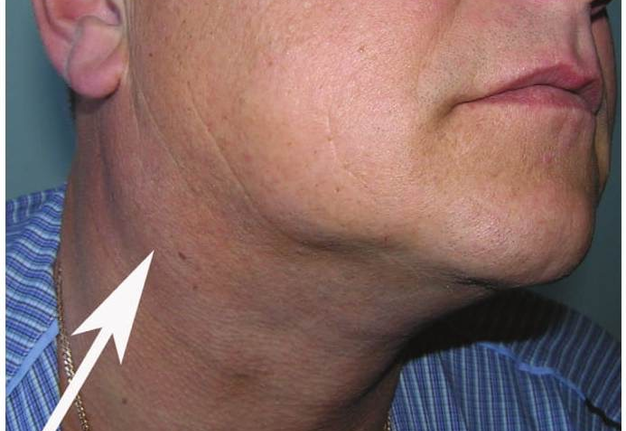
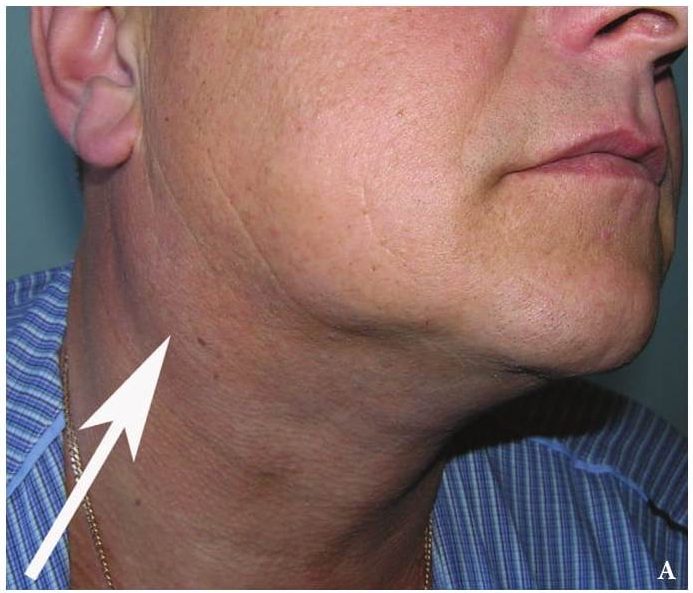
Visually, BCCs shown as a painless limited rounded shape tumor-like lesion with a smooth surface. The skin above it is not changed in color. Do not soldered with surrounding tissues. A compulsory component of the cyst is a lymph node at the inferior pole. Upon swallowing the tumor-like mass does not move (as opposed to thyroglossal duct cysts). Consistency of the cysts are soft-elastic or elastically tense (elastically dense). A fluctuation is may be determined. The BCCs does not cause respiratory and swallowing disorders. Systemic manifestations are not present. With connecting of secondary inflammation the cyst becomes dense, slow-moving, painful, can cause pain upon swallowing, and even talking. The systemic symptoms are (malaise, weakness, fever, etc.) appearing. Puncture of the cyst can get serous-mucous or muco-purulent transparent liquid light brown or dark brown (rare) color. Upon suppuration cyst fluid becomes turbid, pus appears. The skin over the cyst in case of its suppuration becomes hyperemated (Fig 3).
Microscopically desquamated epithelial cells, erythrocytes, lymphocytes, and cholesterol crystals can be detected in a punctate. Upon bacteriological examination a microflora in the content of uncomplicated cysts usually are not found. Only in rare cases low virulent staphylococci or streptococci are founded.
PATHOLOGY
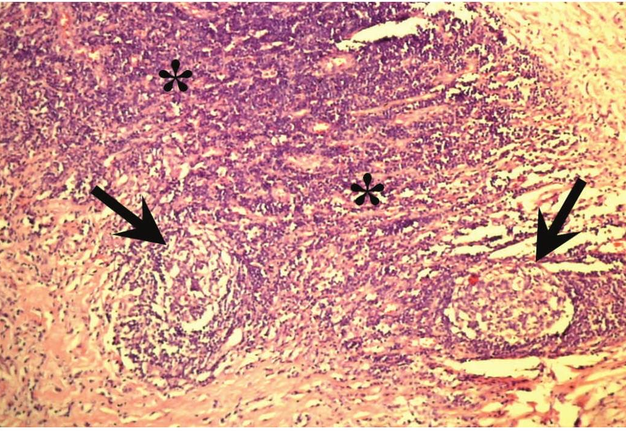
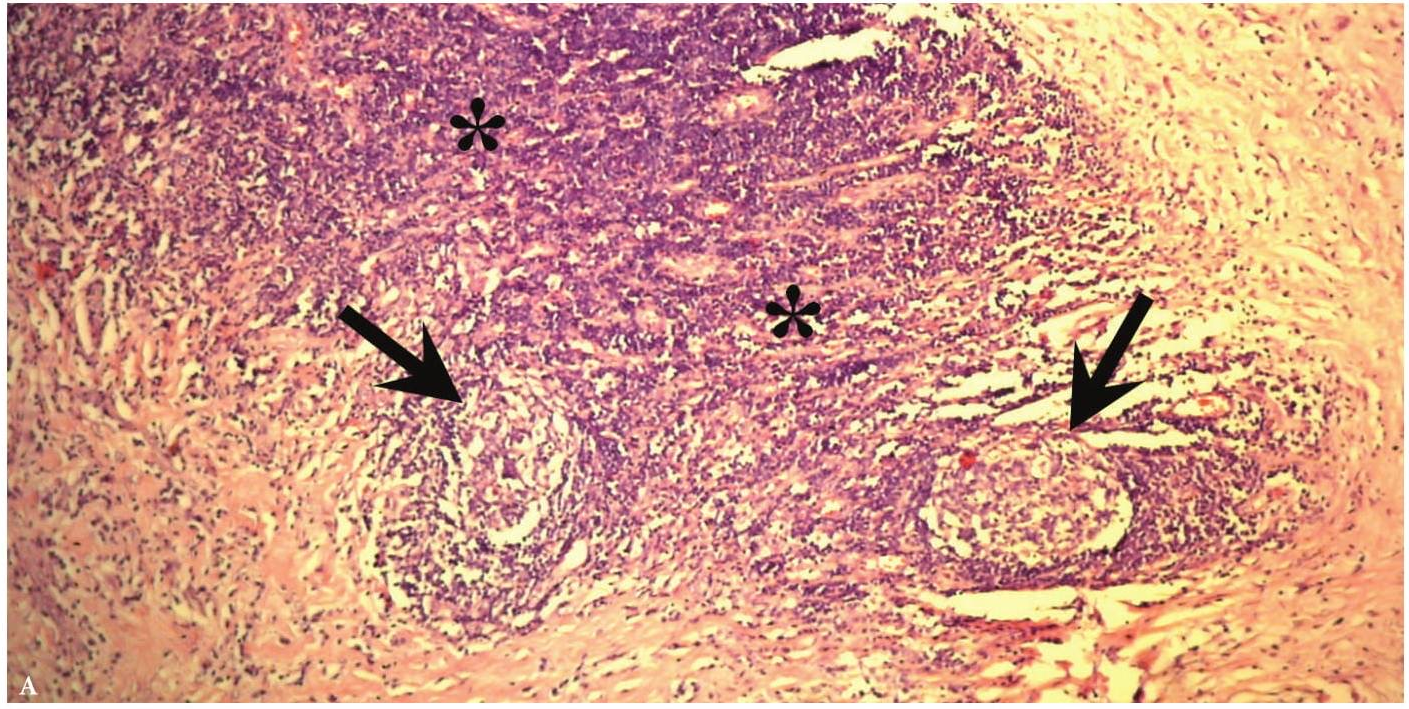
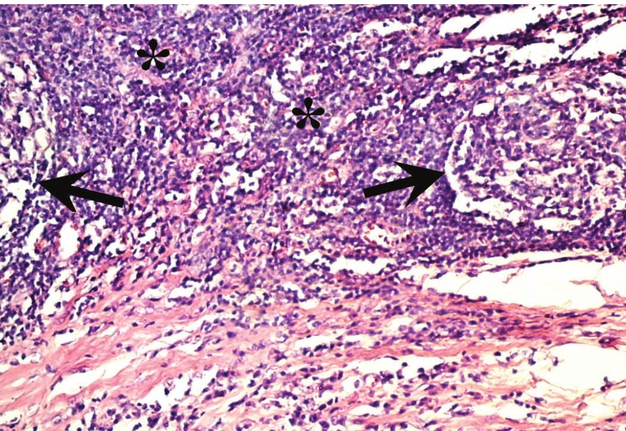
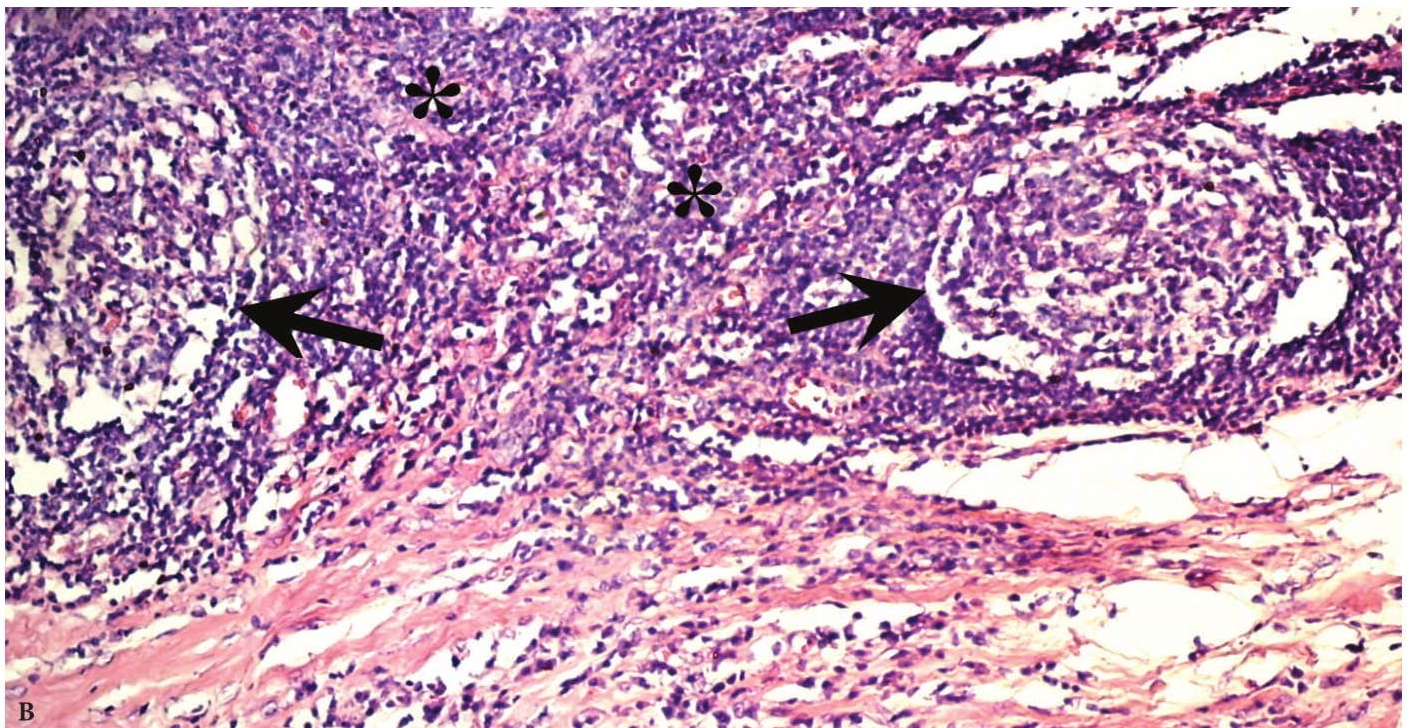
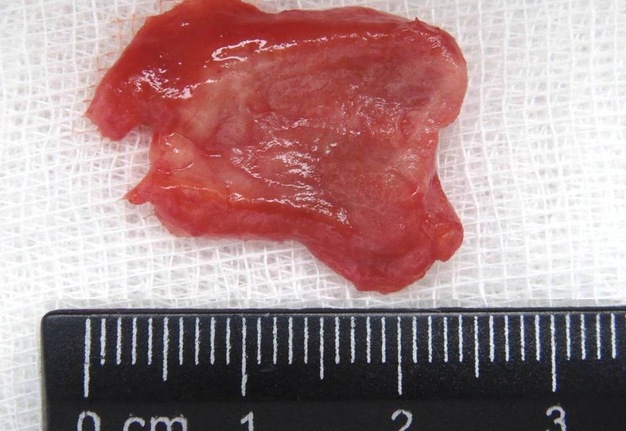
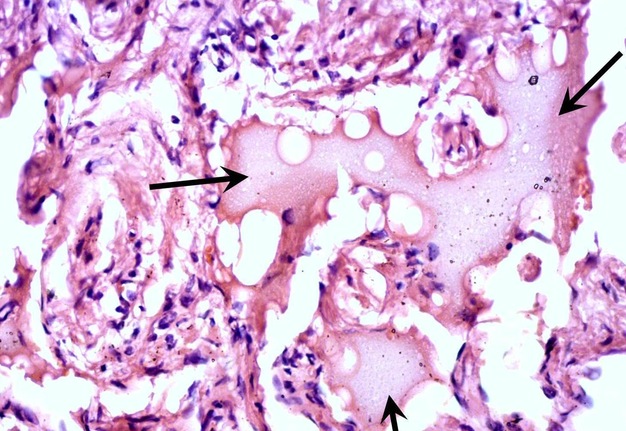
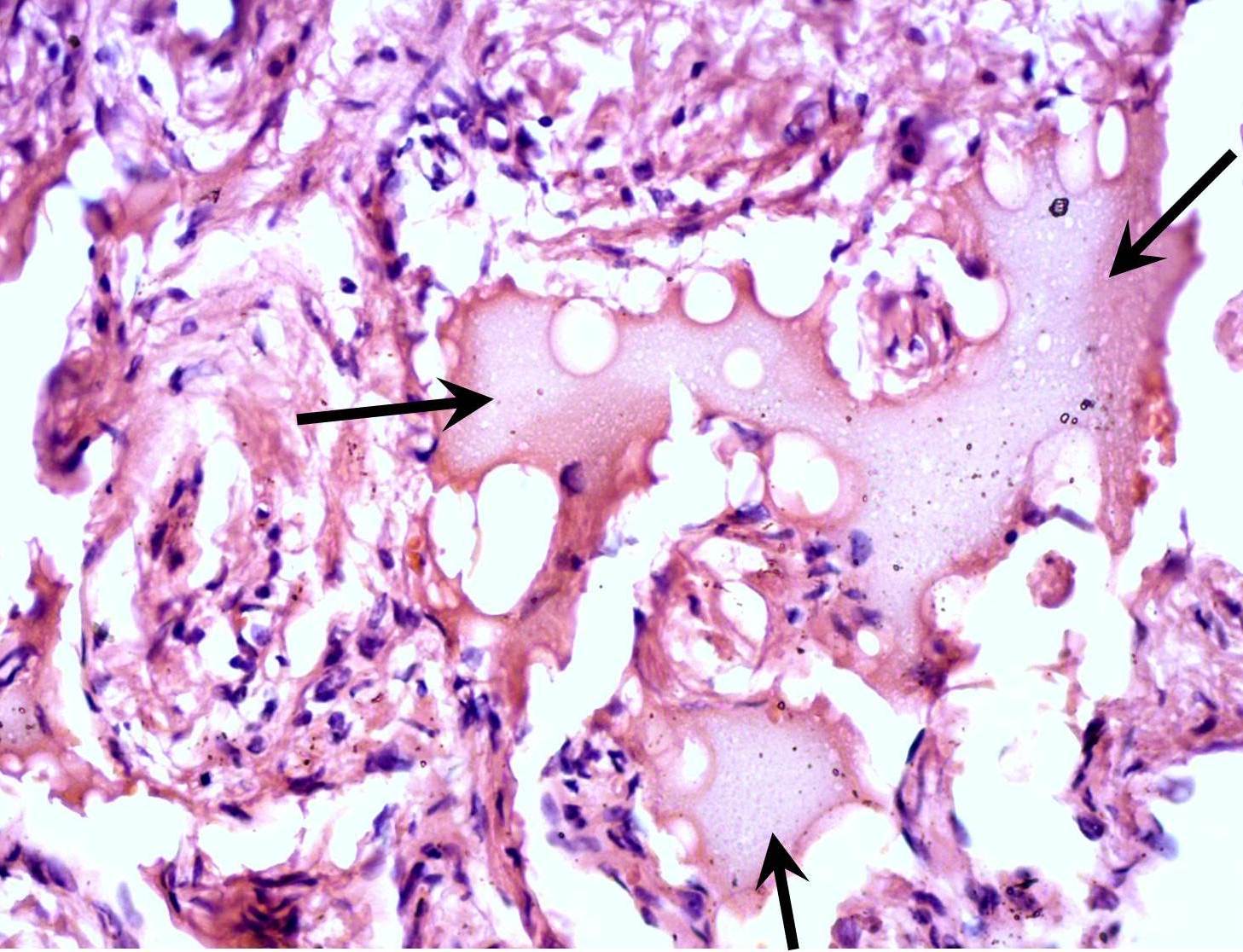
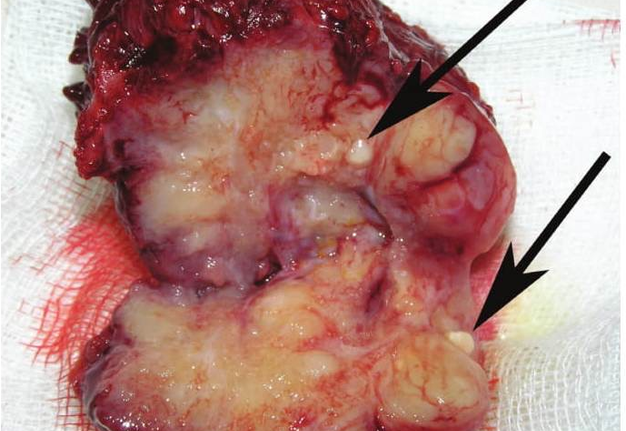
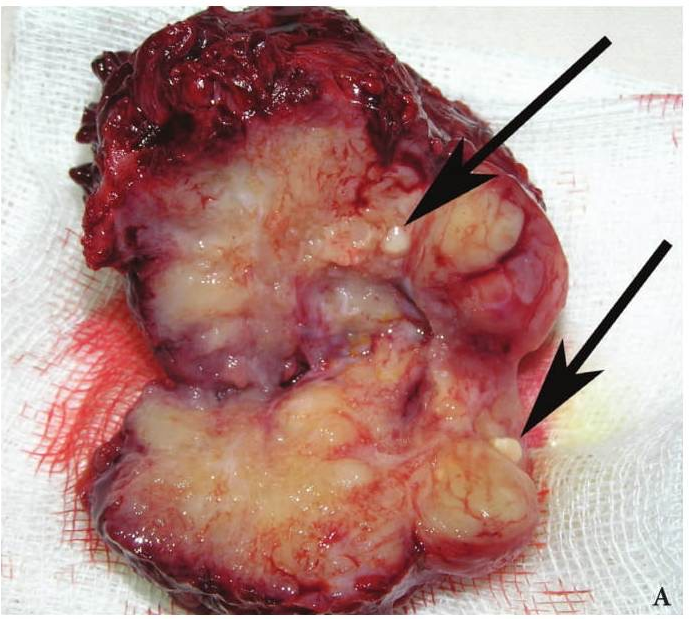
Verification of the diagnosis is provided with aid of pathomorphological investigation. Microscopically the wall of the BCCs consists of a dense connective (fibrous) tissue that is lined with a stratified squamous non-cornified epithelium (ectodermal cysts), and multi-layered columnar epithelium (endodermal cyst). Some BCCs contain ciliated epithelium. In the wall thickness (capsule) is lymphoid tissue, often forms the follicles (germinal centers) (Fig 4) [17]. Significant development of lymphoid tissue suggests that the lateral cysts originate from the branchial apparatus remnants. The inner surface of the cyst may be covered with warty growths of lymphoid tissue (crypts). In its wall identifies the formations like Hassel's corpuscles of thymus gland. Upon suppuration of the cyst the epithelium can partially die and replaced by the connective tissue, there is a thickening of the epithelial lining and its cornification. At the inferior pole of the BCCs the lymph node is often morphologically detected.
In front of the tragus can be found the preauricular (branchial) fistula, which comes from I branchial pocket. The fistulous tract lined by squamous epithelium. Preauricular (tragal) fistulas are often spread deep into the soft tissue to the parotid gland, and even penetrate into it. From these fistulas develop cysts localized in the parotid gland. The morphological difference between these fistulas is that the wall of the fistula, originating from the branchial I pocket have no lymphoid tissue, which is always present in BCCs or fistulas localized in the neck.
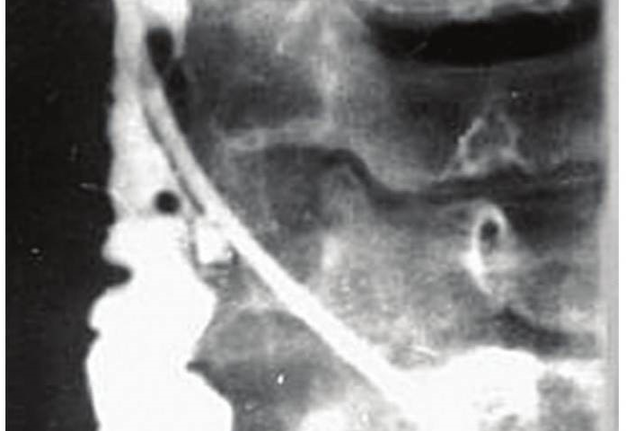
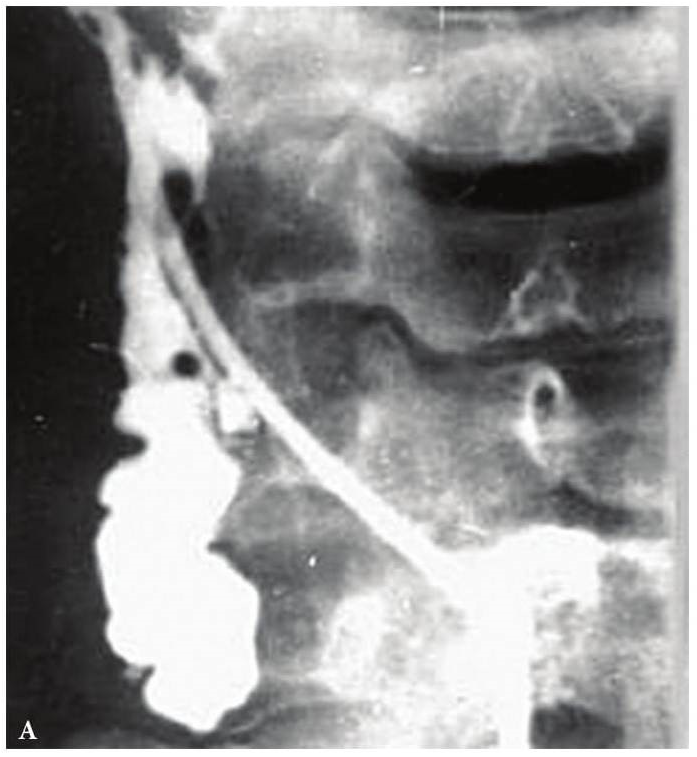
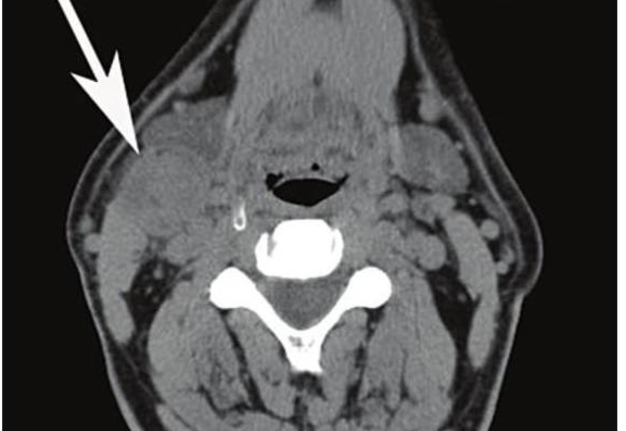
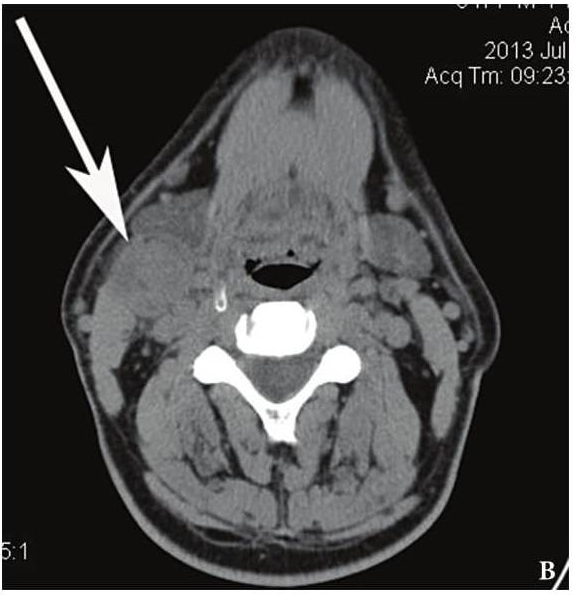
Diagnostics of BCCs is carried out between chronic lymphadenitis (non-specific and specific) [18-21], dermoid (epidermoid) cysts [22, 23], tumors and tumor-like lesions of soft tissues of the neck, blood vessels, nerves and thyroid gland, lymphangiomas (Fig 10) [24-28], metastases of malignant tumors, etc. For more accurate diagnosis the cyst- or fistulography with the administration of radiocontrast agents, CT, MRI, ultrasound can be performed (Fig 5).
ULTRASOUND
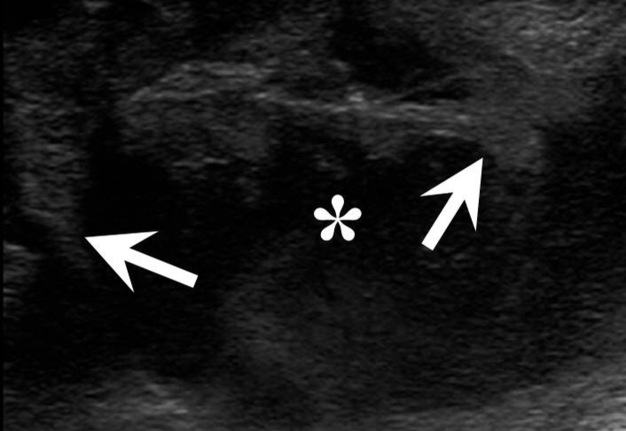
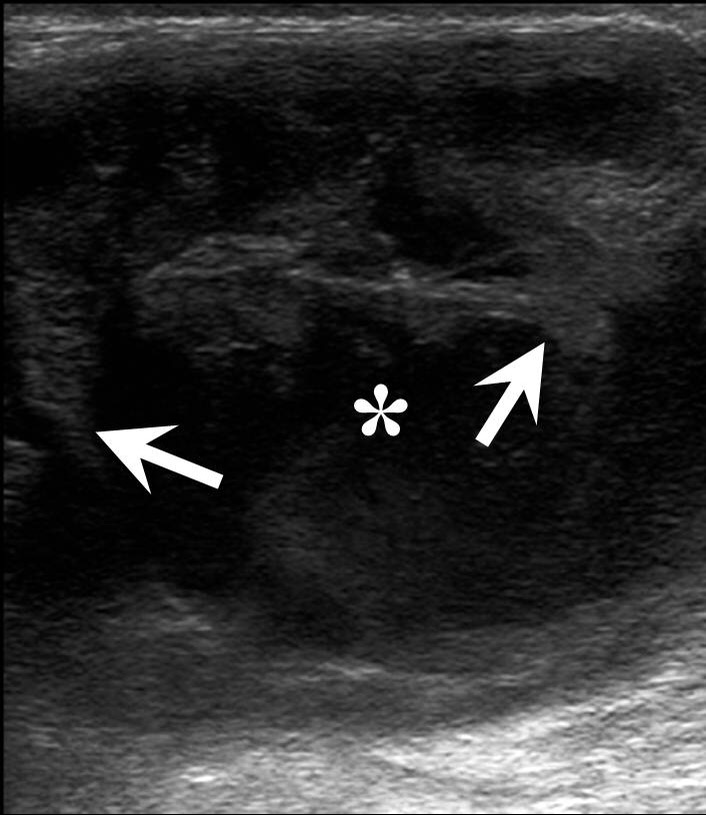
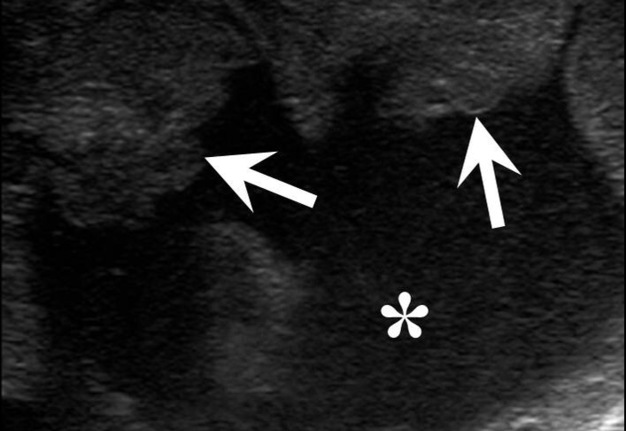
Upon ultrasound diagnostics are estimated: the location of the lesion, its size, wall thickness and the presence of septations, edges, borders, internal echogenicity, presence of acoustic enhancement artifact, fistula, vascularization at Doppler ultrasound. Compression of the mass by ultrasonic transducer confirms the true cystic nature helping in differential diagnosis. BCCs at ultrasonography visualized as cystic mass of oval or round shape with smooth surface (Figs 6-8). According to Ahuja A.T. et al. (2000) [19] distinguish four echogenicity patterns of BCCs content: truly anehoic (41%), predominantly homogeneously hypoechoic but showed the presence of internal, low-amplitude, freely floating debris (diagnosed at 24%), hyperechoic with pseudosolid appearance (12%), heterogeneous internal echoes with internal debris and septa (23%). On echogenicity type affects the consistency of the content of the lateral cyst, which may vary depending on the presence of inflammatory processes (liquid-cystic, cystic-liquid with debris, pasty, suppurated). Pseudosolid appearance due to the presence of the protein content of the cysts produced by the epithelial lining [31]. On color and power Doppler ultrasound BCCs are avascular. Cyst wall shows as hyper- or isoehoic linear structure, often avascular at Doppler ultrasound. The thickness of the wall may vary in different parts of cysts and reach 1.0cm in recurrent inflammations [30, 31], but it is also possible thinning, i.e. becomes non-differentiable [4].
CT & MRI
According to Weerakkody Y., Gaillard F. et al. on the CT and MRI images the BCCs have the following features.
For the patients with BCCs is recommended to perform magnetic resonance imaging in three modes. On T1-weighted MR images BCCs shows as a variable signal depending on the protein content. If their content is high – high-intensity signal, low – low intensity. On T2-weighted MR images BCCs are usually high intensity. On contrast-enhanced T1-weighted MRI, in uncomplicated cases, the BCCs have no enhancement.
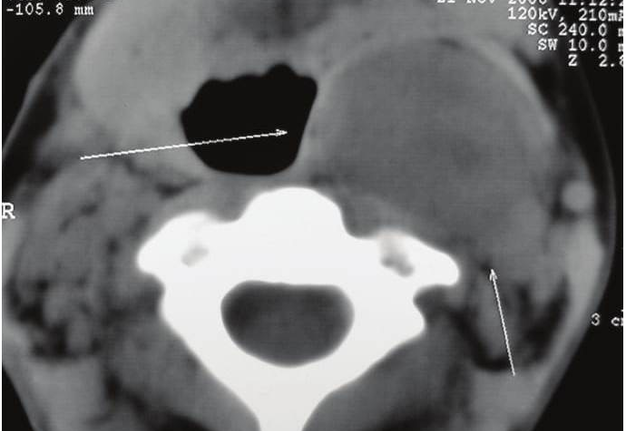
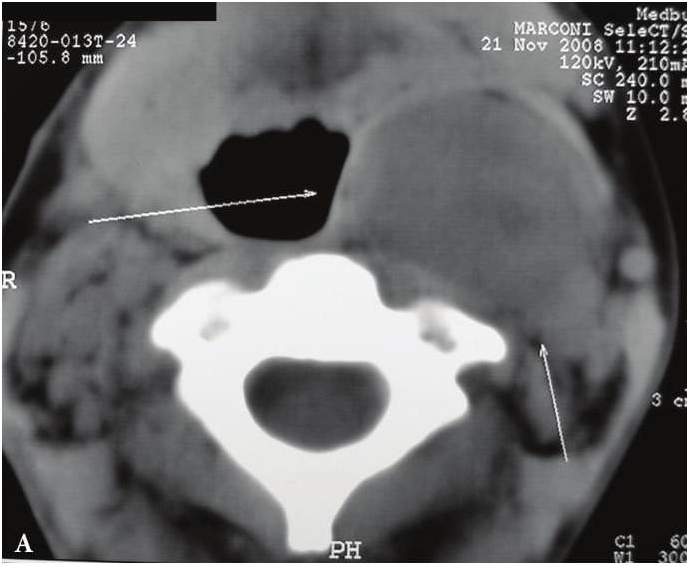
On contrast-enhanced multidetector CT images BCCs are spherical or round shape, its walls are clearly distinguished from the surrounding tissue. The wall thickness varies from 0.1 to 1.0 cm. The cystic wall may penetrate between internal and external carotid arteries, in the region above the bifurcation of the common carotid artery (scraps symptom or beak tail) [6]. The density of the contents of the cavities (depending on the type of content and the presence of inflammation) ranges from 10 to +27,8 (± 6,0) HU, wall density is up to +102,0 (± 8,0) HU. Hounsfield units (HU) is a units of measure indicating the absorption of the X-rays by various tissues of the body. Remember that most absorbs x-rays the tooth enamel (3000 HU) and cortical bone (from 850 to 2000 HU), least of all − the blood (20-70 HU) and muscle (10-70 HU), adipose tissue (from −40 to −100 HU).
BCCs should be differentiated with esophageal diverticula. Esophageal diverticula presented as a round shape lesion, which is located in front of sternocleidomastoid muscle. The lesion is soft or pastry to the touch, collapses on palpation and transmits peristaltic waves during swallowing. With eating it is filled, and increases in size. The pain is intensified with filling of diverticula after eating. Swallowing can be painful, especially during exacerbation of the inflammatory process.
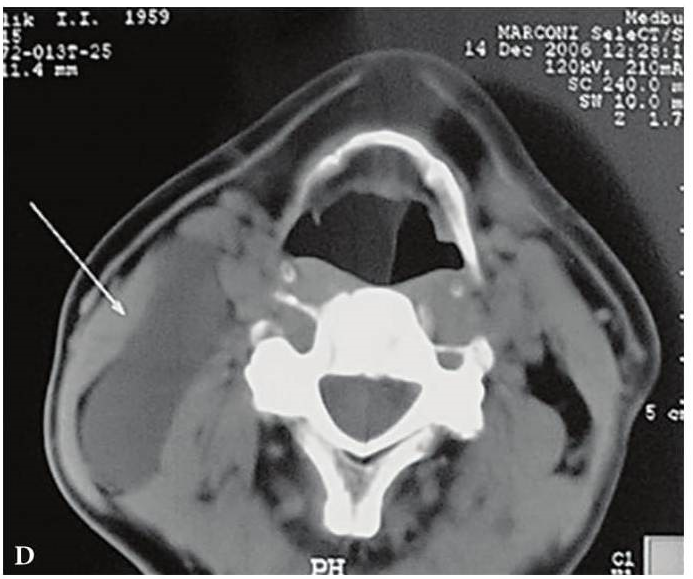
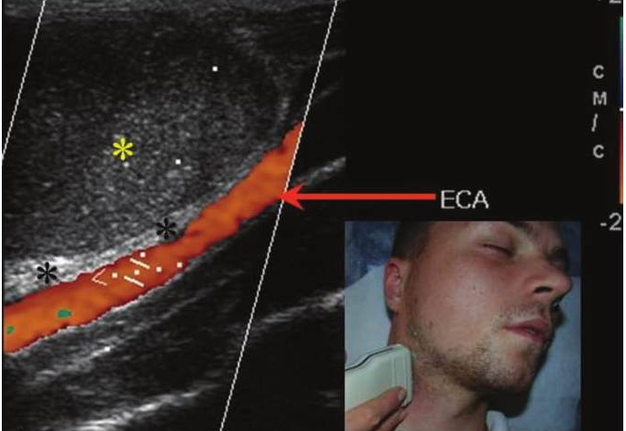
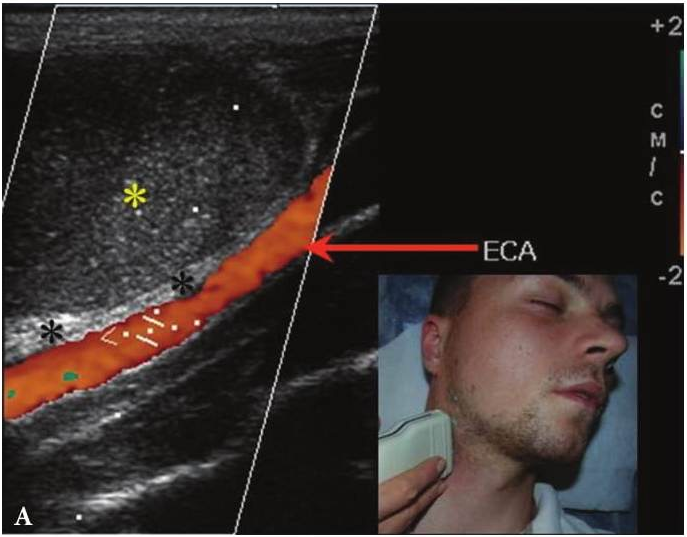
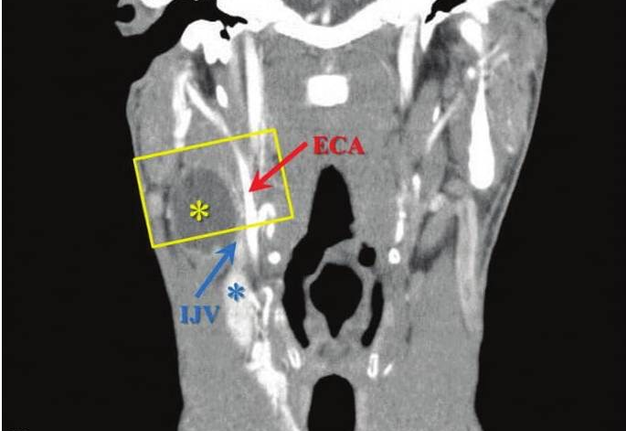
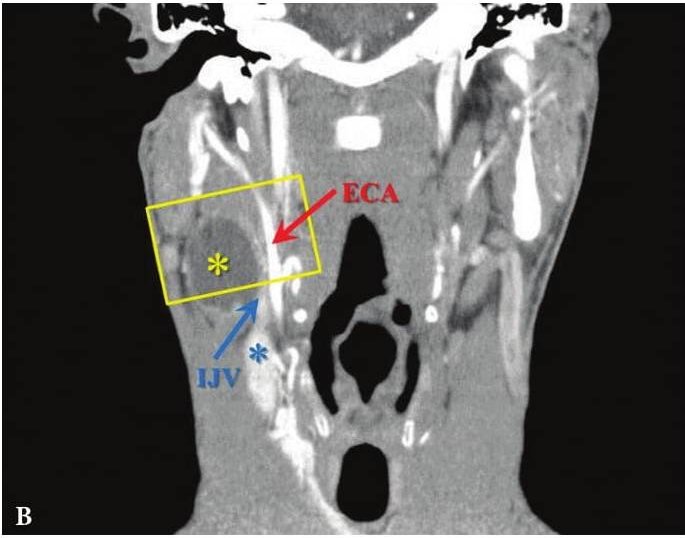
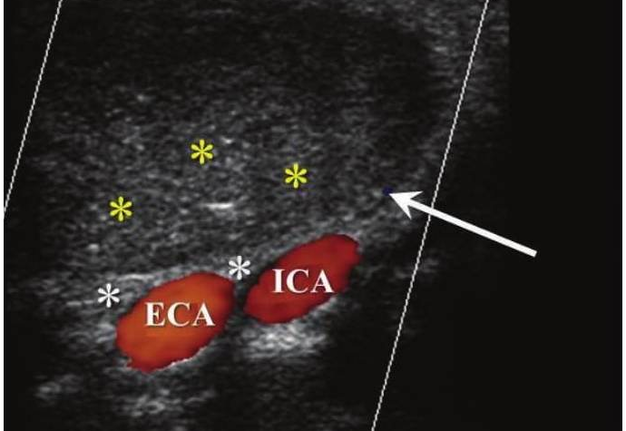
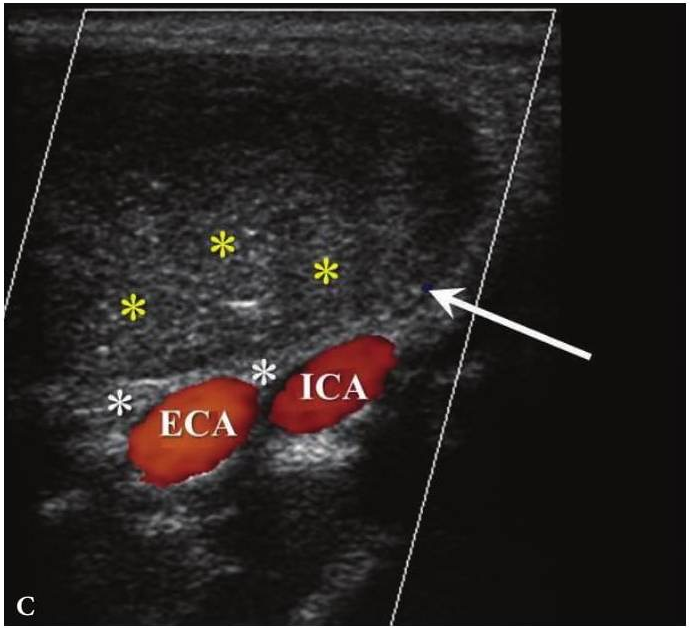
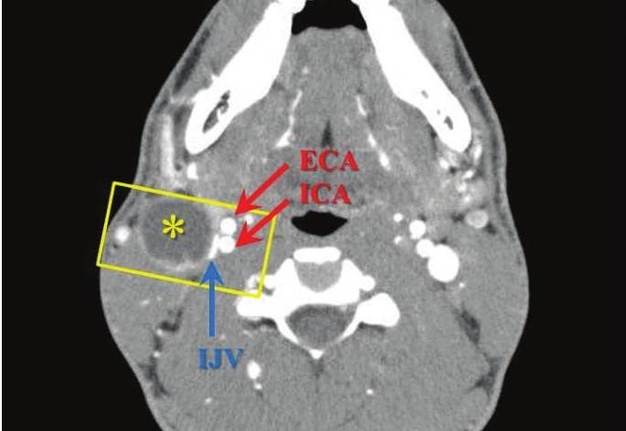
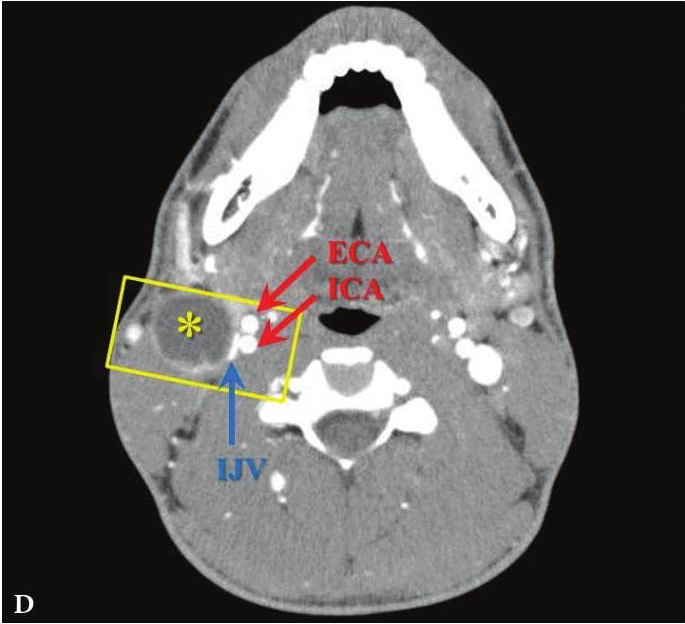
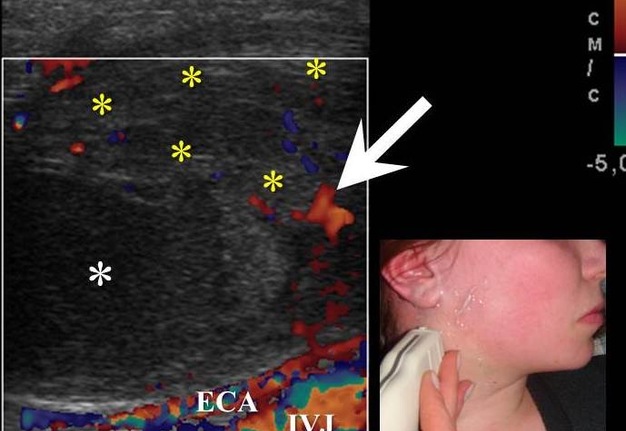
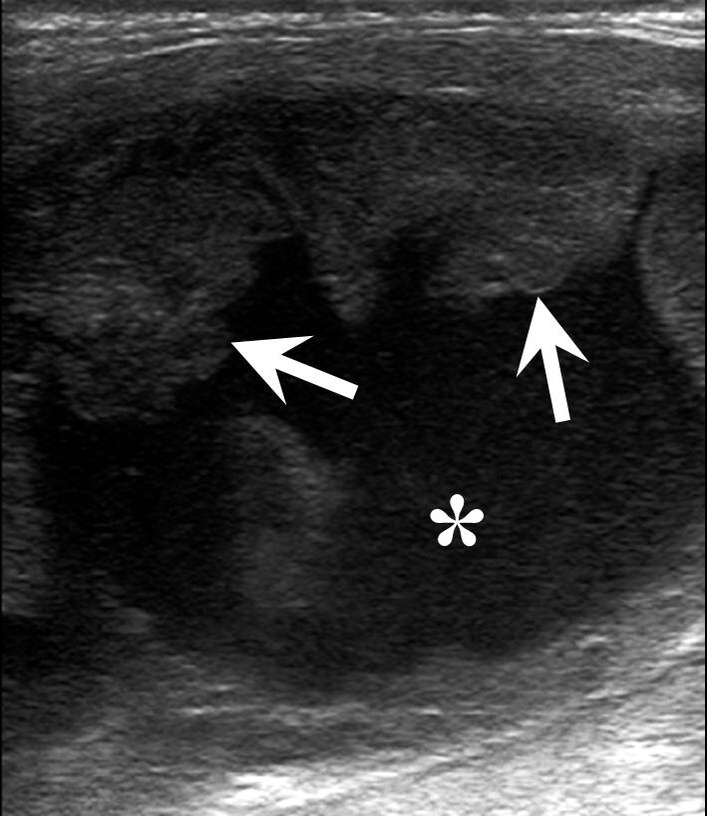
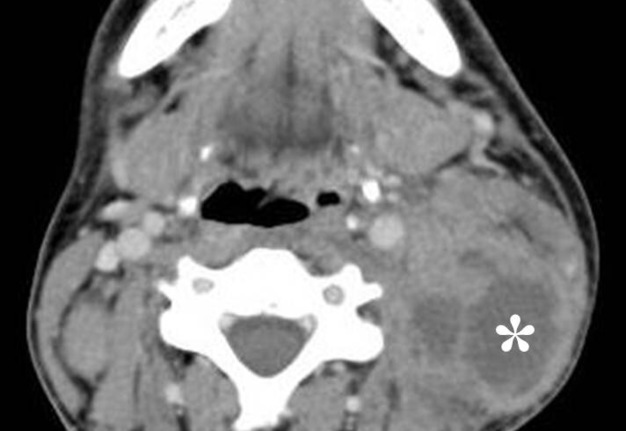
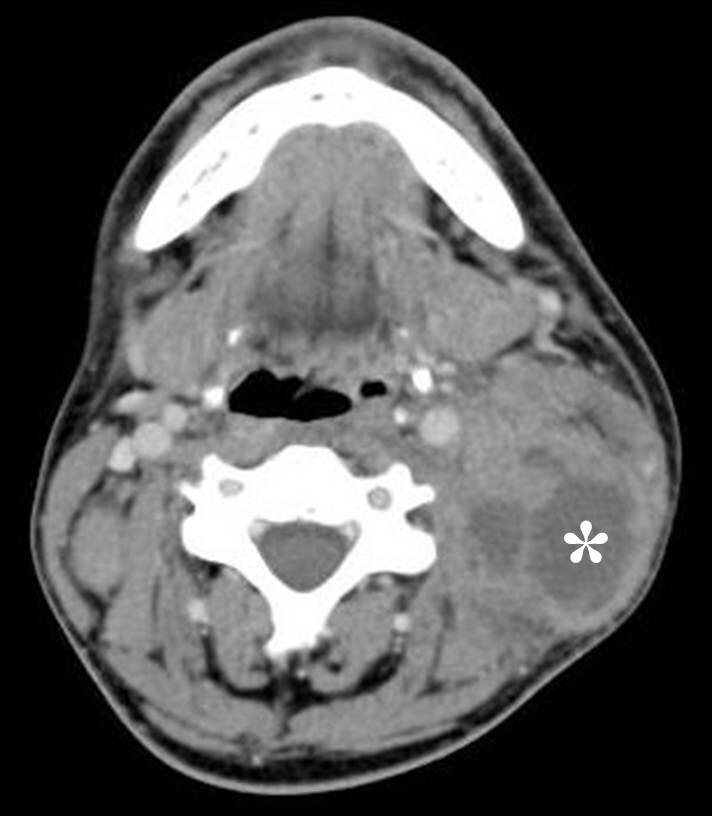
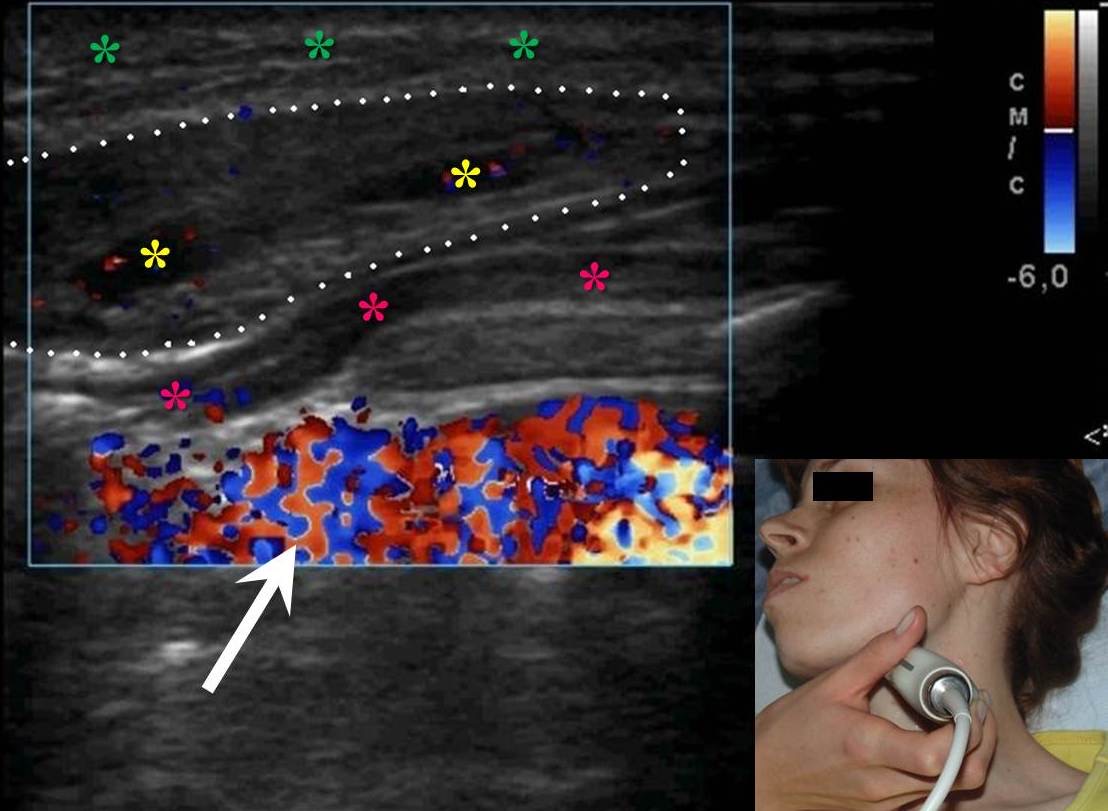
FIGURE 6A. BCC in a 19-years-old patient. Longitudinal spectral Doppler sonogram (A) performed by linear transducer shows oval shaped cystic lesion with smooth margins, sharp edges, filled with a heterogeneous content (yellow asterisks) which simulate “pseudosolid” appearance. Note that cyst is adjacent to the external carotid artery (ECA) which is marked by a red arrow. Acoustic enhancement artifact (black asterisks) is distal to the cyst. Cyst is avascular. On contrast-enhanced CT (B) is confirmed the presence of cystic lesion (yellow aterisk) adjacent to the external carotid artery (ECA) and compression of the internal jugular vein (IJV). By yellow frame is marked the sonogram location performed at Figure 6A. Transverse spectral Doppler ultrasound (C) performed by linear transducer shows oval shaped cystic lesion with smooth contours, sharp edges, filled by heterogeneous content (yellow asterisks) which create “pseudosolid” appearance. The cyst is adjacent to the external (ECA) and internal carotid artery (ICA), and internal jugular vein (IJV), squeezing it (white arrow). Artifact of posterior acoustic enhancement (white asterisks) visualized distal to the cyst. Blood flow within the lesion and its wall is absent. On contrast CT image (D) confirmed the presence of cystic lesion (yellow asterisk) adjacent to the carotid arteries (ECA, ICA) and compression of the internal jugular vein (IJV). The density of the cyst content is +27,8 (± 6,0) HU. Yellow frame marks the position of a sonogram obtained at Figure 6C.
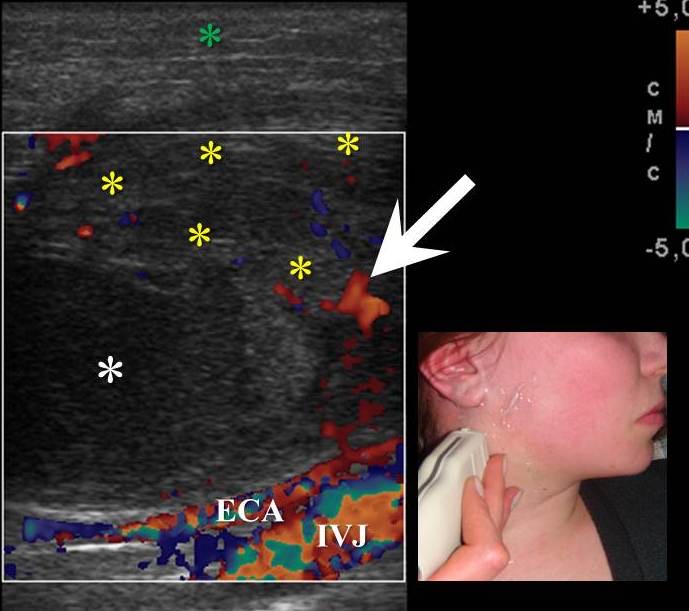
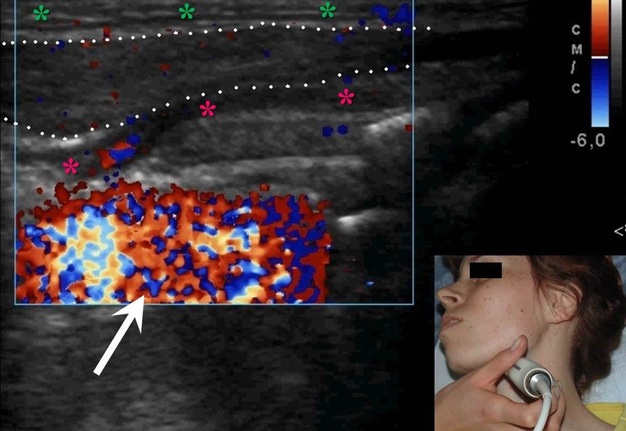
FIGURE 6B. BCC in a 19-years-old patient. Longitudinal spectral Doppler sonogram (A) performed by linear transducer shows oval shaped cystic lesion with smooth margins, sharp edges, filled with a heterogeneous content (yellow asterisks) which simulate “pseudosolid” appearance. Note that cyst is adjacent to the external carotid artery (ECA) which is marked by a red arrow. Acoustic enhancement artifact (black asterisks) is distal to the cyst. Cyst is avascular. On contrast-enhanced CT (B) is confirmed the presence of cystic lesion (yellow aterisk) adjacent to the external carotid artery (ECA) and compression of the internal jugular vein (IJV). By yellow frame is marked the sonogram location performed at Figure 6A. Transverse spectral Doppler ultrasound (C) performed by linear transducer shows oval shaped cystic lesion with smooth contours, sharp edges, filled by heterogeneous content (yellow asterisks) which create “pseudosolid” appearance. The cyst is adjacent to the external (ECA) and internal carotid artery (ICA), and internal jugular vein (IJV), squeezing it (white arrow). Artifact of posterior acoustic enhancement (white asterisks) visualized distal to the cyst. Blood flow within the lesion and its wall is absent. On contrast CT image (D) confirmed the presence of cystic lesion (yellow asterisk) adjacent to the carotid arteries (ECA, ICA) and compression of the internal jugular vein (IJV). The density of the cyst content is +27,8 (± 6,0) HU. Yellow frame marks the position of a sonogram obtained at Figure 6C.
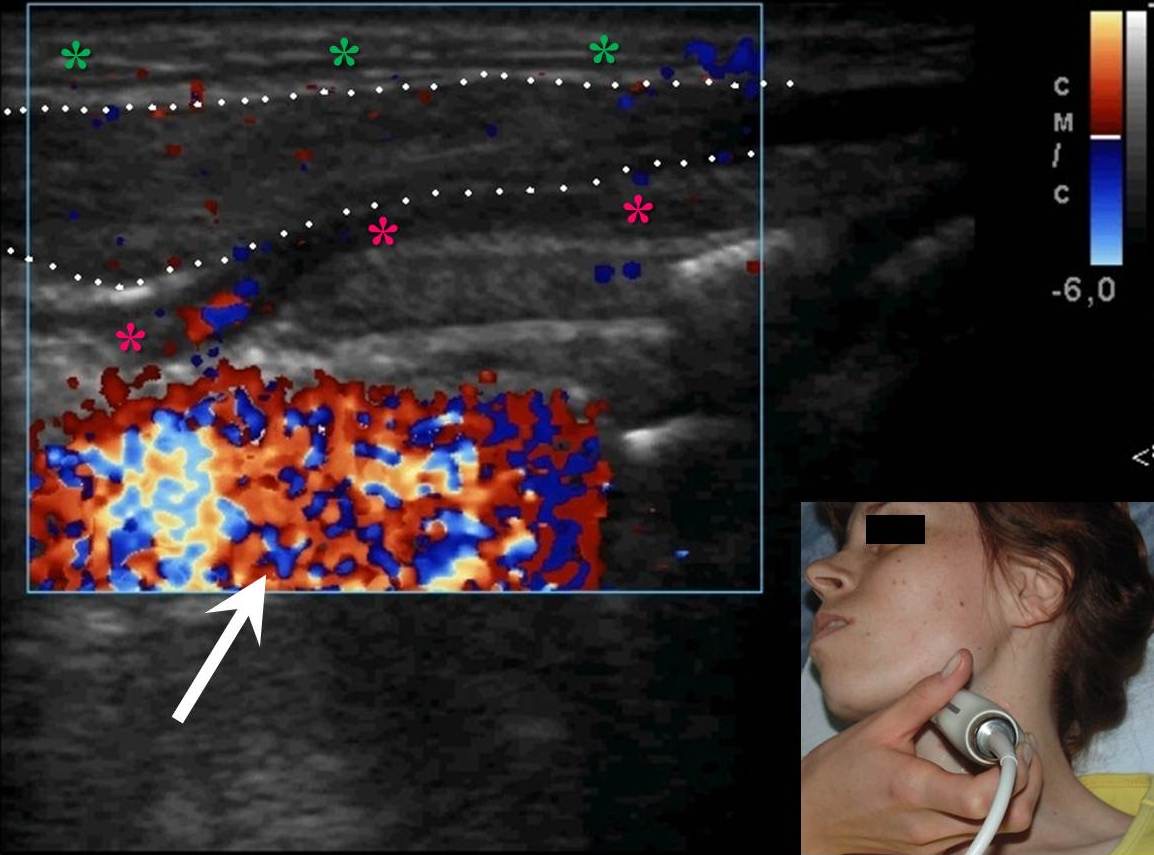
FIGURE 6C. BCC in a 19-years-old patient. Longitudinal spectral Doppler sonogram (A) performed by linear transducer shows oval shaped cystic lesion with smooth margins, sharp edges, filled with a heterogeneous content (yellow asterisks) which simulate “pseudosolid” appearance. Note that cyst is adjacent to the external carotid artery (ECA) which is marked by a red arrow. Acoustic enhancement artifact (black asterisks) is distal to the cyst. Cyst is avascular. On contrast-enhanced CT (B) is confirmed the presence of cystic lesion (yellow aterisk) adjacent to the external carotid artery (ECA) and compression of the internal jugular vein (IJV). By yellow frame is marked the sonogram location performed at Figure 6A. Transverse spectral Doppler ultrasound (C) performed by linear transducer shows oval shaped cystic lesion with smooth contours, sharp edges, filled by heterogeneous content (yellow asterisks) which create “pseudosolid” appearance. The cyst is adjacent to the external (ECA) and internal carotid artery (ICA), and internal jugular vein (IJV), squeezing it (white arrow). Artifact of posterior acoustic enhancement (white asterisks) visualized distal to the cyst. Blood flow within the lesion and its wall is absent. On contrast CT image (D) confirmed the presence of cystic lesion (yellow asterisk) adjacent to the carotid arteries (ECA, ICA) and compression of the internal jugular vein (IJV). The density of the cyst content is +27,8 (± 6,0) HU. Yellow frame marks the position of a sonogram obtained at Figure 6C.
FIGURE 6D. BCC in a 19-years-old patient. Longitudinal spectral Doppler sonogram (A) performed by linear transducer shows oval shaped cystic lesion with smooth margins, sharp edges, filled with a heterogeneous content (yellow asterisks) which simulate “pseudosolid” appearance. Note that cyst is adjacent to the external carotid artery (ECA) which is marked by a red arrow. Acoustic enhancement artifact (black asterisks) is distal to the cyst. Cyst is avascular. On contrast-enhanced CT (B) is confirmed the presence of cystic lesion (yellow aterisk) adjacent to the external carotid artery (ECA) and compression of the internal jugular vein (IJV). By yellow frame is marked the sonogram location performed at Figure 6A. Transverse spectral Doppler ultrasound (C) performed by linear transducer shows oval shaped cystic lesion with smooth contours, sharp edges, filled by heterogeneous content (yellow asterisks) which create “pseudosolid” appearance. The cyst is adjacent to the external (ECA) and internal carotid artery (ICA), and internal jugular vein (IJV), squeezing it (white arrow). Artifact of posterior acoustic enhancement (white asterisks) visualized distal to the cyst. Blood flow within the lesion and its wall is absent. On contrast CT image (D) confirmed the presence of cystic lesion (yellow asterisk) adjacent to the carotid arteries (ECA, ICA) and compression of the internal jugular vein (IJV). The density of the cyst content is +27,8 (± 6,0) HU. Yellow frame marks the position of a sonogram obtained at Figure 6C.
FIGURE 7. An infected BCC in a 18-years-old female. Longitudinal color Doppler ultrasound shows cystic oval shape lesion with hypoechoic content (white asterisk). Note inflammatory hyperemia of sternocleidomastoid muscle (yellow asterisks) in a form of its increased vascularity (arrow). Edema, decreased echogenicity of the surrounded tissues is marked by green asterisk. Lesion is avascular, adjacent to the neurovascular bundle of the neck. External carotid artery and internal jugular vein are marked by ECA and IJV.
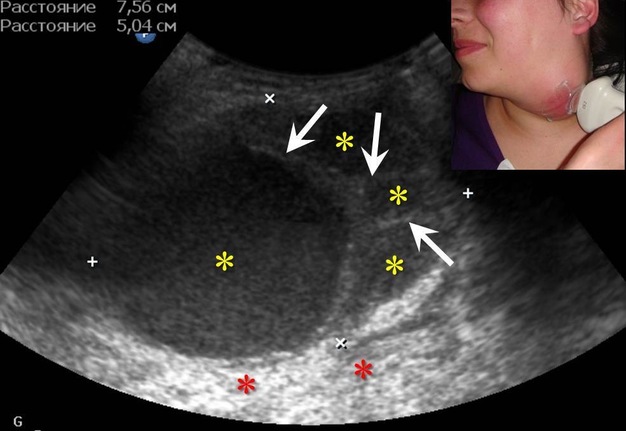
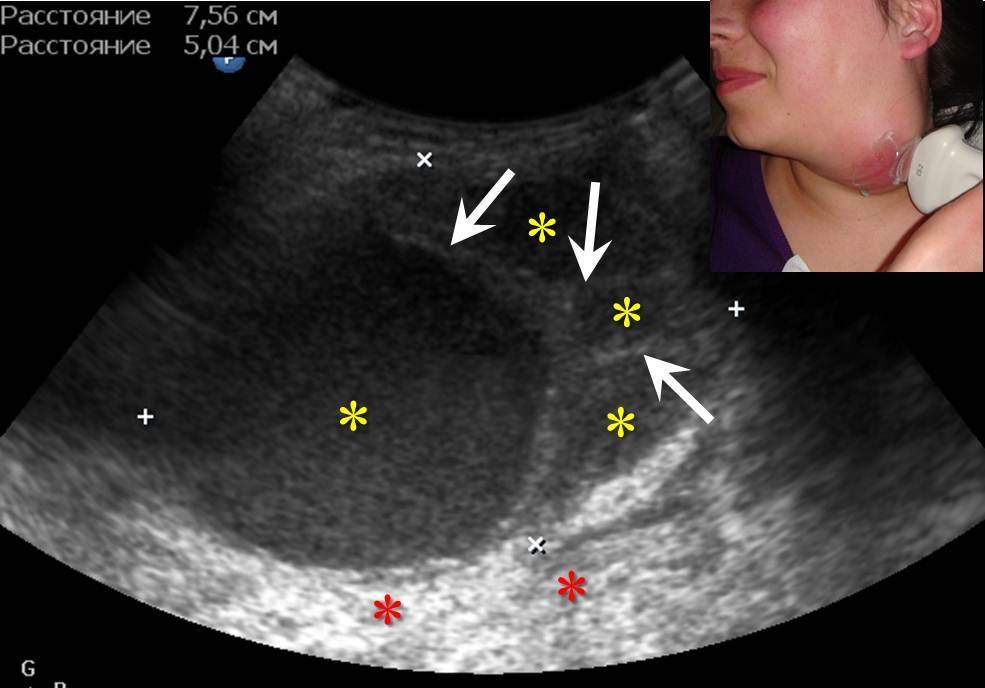
FIGURE 8A. Suppurated multicameral BCC in a 33-years-old female. A sonogram (A) performed by convex transducer shows a cystic lesion (its size are marked with “+” and “x” are equal 7.5- х 5.0- cm) of the left neck with the presence of isoechoic septations (arrows). Anechoic cyst content in cameras are indicated by yellow asterisks, an artifact of acoustic enhancement – by red asterisks. An axial MDCT scan (B) confirms the presence of intracystic septations (arrow). The density of the cystic content is equal to +10, +15 HU. Cameras are marked by asterisks.
FIGURE 8B. Suppurated multicameral BCC in a 33-years-old female. A sonogram (A) performed by convex transducer shows a cystic lesion (its size are marked with “+” and “x” are equal 7.5- х 5.0- cm) of the left neck with the presence of isoechoic septations (arrows). Anechoic cyst content in cameras are indicated by yellow asterisks, an artifact of acoustic enhancement – by red asterisks. An axial MDCT scan (B) confirms the presence of intracystic septations (arrow). The density of the cystic content is equal to +10, +15 HU. Cameras are marked by asterisks.
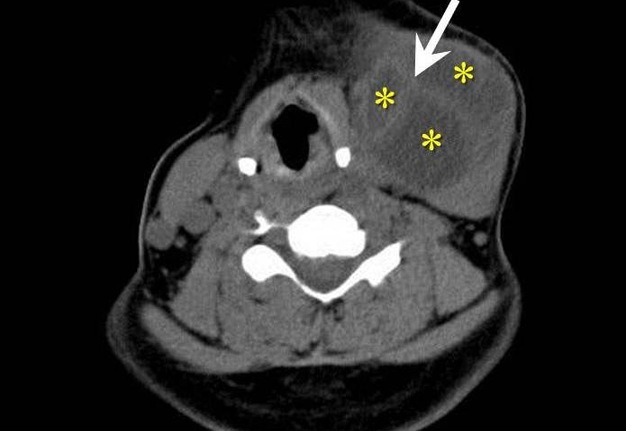
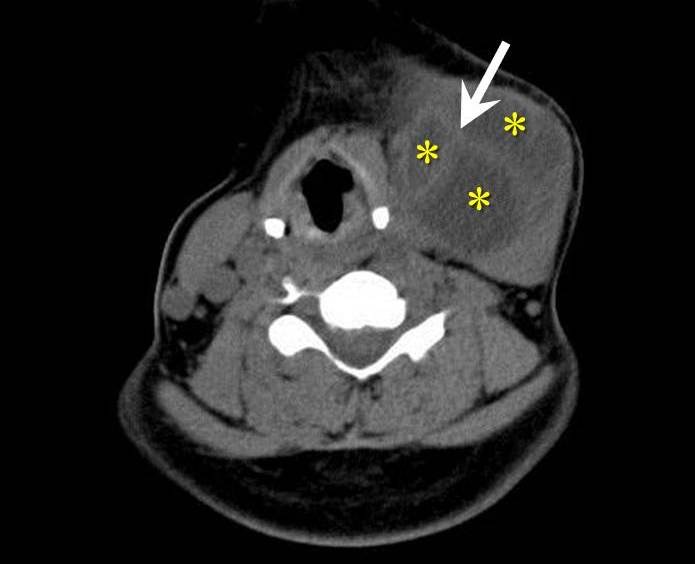
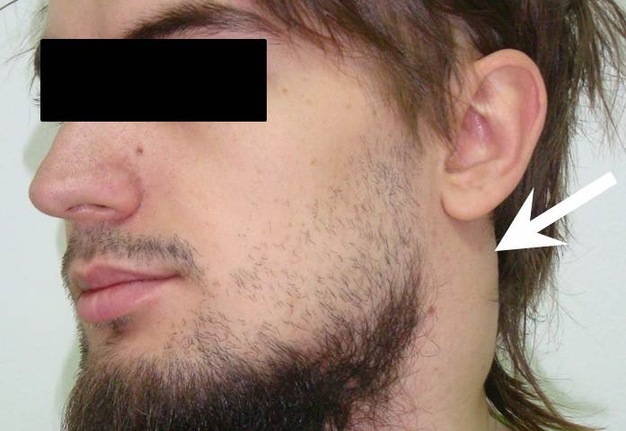
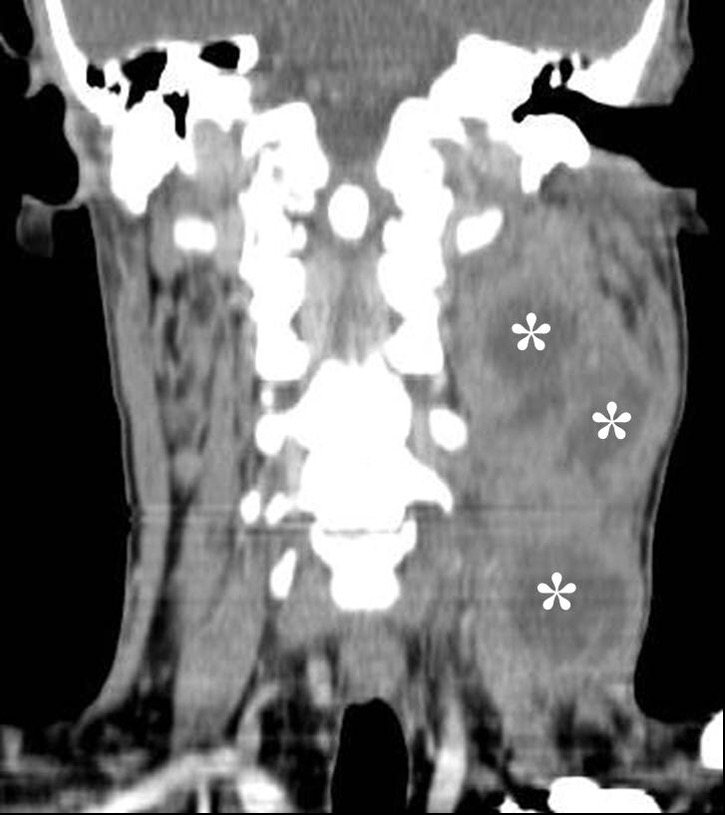
FIGURE 9A. A 24-years-old man with cystic squamous cell carcinoma of the neck (poorly differentiated, which have the most aggressive behavior). Clinical photograph of the patient (А). Transverse gray scale ultrasound of the lower (B) and upper (C) neck shows multicameral lesion with anechoic cystic (asterisks) and heterogenous solid component, presented in the form of irregularly shaped intracystic growths (arrows). Acoustic enhancement artifact is presented. On contrast-enhanced CT images (D, E), the lesion on left side of the neck is multicameral (cameras are marked by asterisks) with solid component accumulating contrast.
FIGURE 9B. A 24-years-old man with cystic squamous cell carcinoma of the neck (poorly differentiated, which have the most aggressive behavior). Clinical photograph of the patient (А). Transverse gray scale ultrasound of the lower (B) and upper (C) neck shows multicameral lesion with anechoic cystic (asterisks) and heterogenous solid component, presented in the form of irregularly shaped intracystic growths (arrows). Acoustic enhancement artifact is presented. On contrast-enhanced CT images (D, E), the lesion on left side of the neck is multicameral (cameras are marked by asterisks) with solid component accumulating contrast.
FIGURE 9C. A 24-years-old man with cystic squamous cell carcinoma of the neck (poorly differentiated, which have the most aggressive behavior). Clinical photograph of the patient (А). Transverse gray scale ultrasound of the lower (B) and upper (C) neck shows multicameral lesion with anechoic cystic (asterisks) and heterogenous solid component, presented in the form of irregularly shaped intracystic growths (arrows). Acoustic enhancement artifact is presented. On contrast-enhanced CT images (D, E), the lesion on left side of the neck is multicameral (cameras are marked by asterisks) with solid component accumulating contrast.
FIGURE 9D. A 24-years-old man with cystic squamous cell carcinoma of the neck (poorly differentiated, which have the most aggressive behavior). Clinical photograph of the patient (А). Transverse gray scale ultrasound of the lower (B) and upper (C) neck shows multicameral lesion with anechoic cystic (asterisks) and heterogenous solid component, presented in the form of irregularly shaped intracystic growths (arrows). Acoustic enhancement artifact is presented. On contrast-enhanced CT images (D, E), the lesion on left side of the neck is multicameral (cameras are marked by asterisks) with solid component accumulating contrast.
FIGURE 9E. A 24-years-old man with cystic squamous cell carcinoma of the neck (poorly differentiated, which have the most aggressive behavior). Clinical photograph of the patient (А). Transverse gray scale ultrasound of the lower (B) and upper (C) neck shows multicameral lesion with anechoic cystic (asterisks) and heterogenous solid component, presented in the form of irregularly shaped intracystic growths (arrows). Acoustic enhancement artifact is presented. On contrast-enhanced CT images (D, E), the lesion on left side of the neck is multicameral (cameras are marked by asterisks) with solid component accumulating contrast.
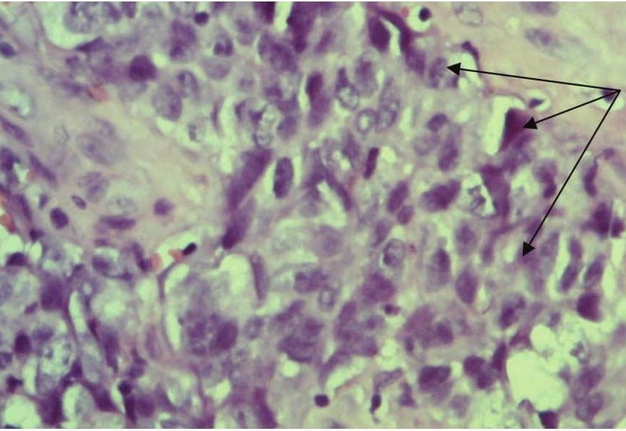
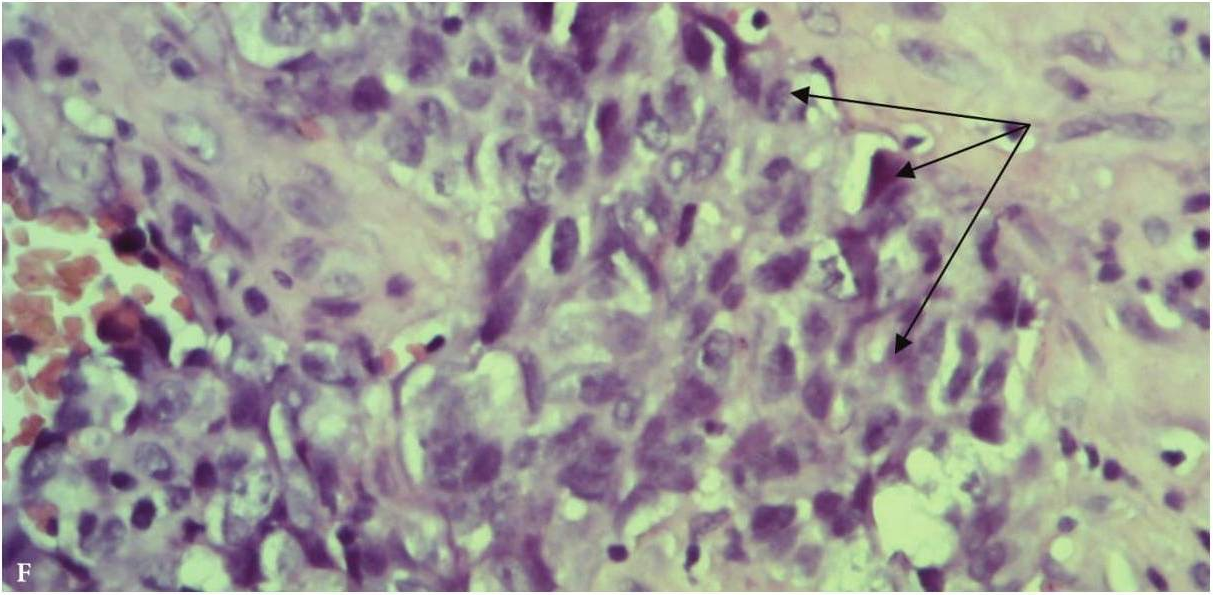
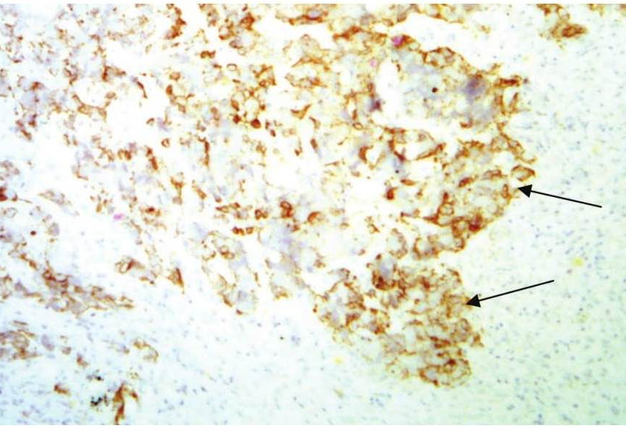
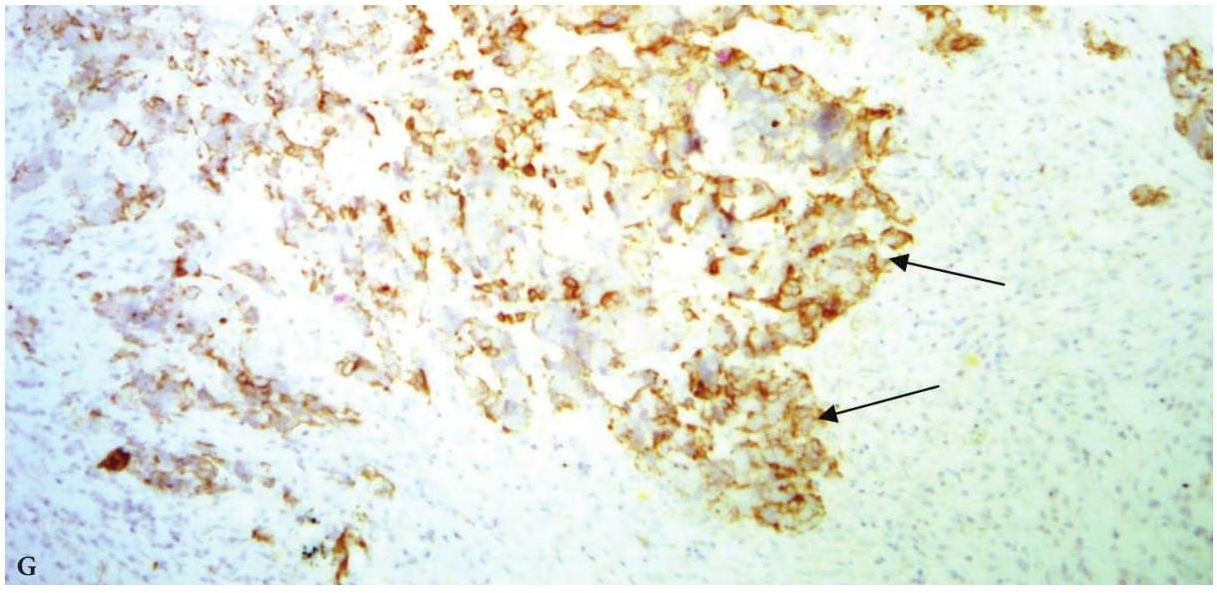
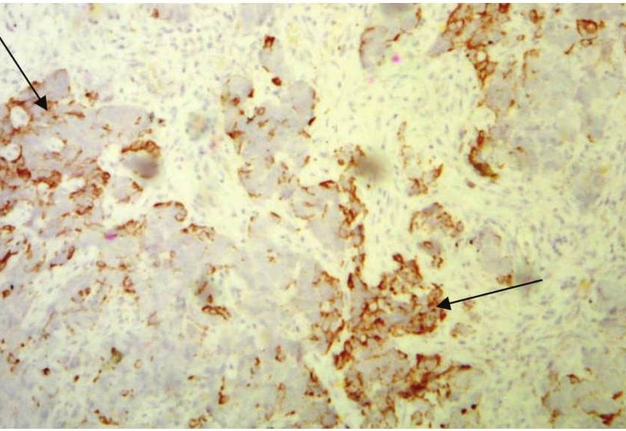
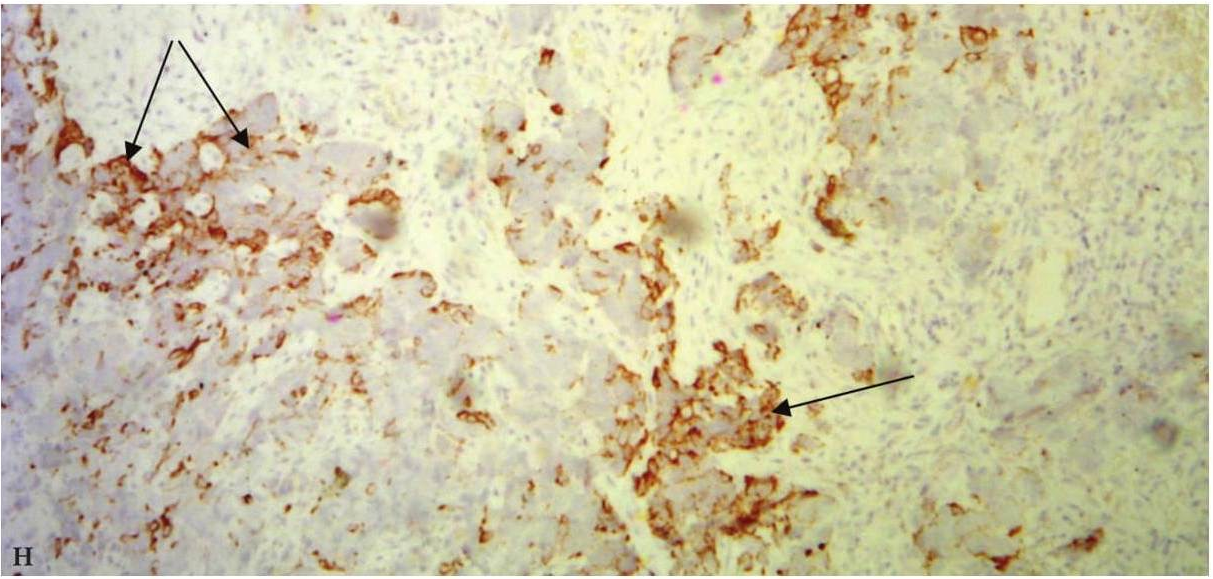
FIGURE 9F. Trephine biopsy histology (F) shows, that in fragment of the lymph node tissue with presence of fibrous tissue, the growth of poorly differentiated malignant tumor (arrows) of epithelial nature with extensive necrosis in the tumor and lymph node tissue, hemorrhages is determined (hematoxylin and eosin; magnification x200). Immunohistochemistry (G, H) shows positive membrane reaction (arrows) with Cytokeratin AE1/AE3 and Cytokeratin 5/6. Reaction with CD45, S100 is negative (reaction with CD45- is determined in intact cells of the lymph node tissue). Thus, there is metastasis of poorly differentiated squamous cell carcinoma G3 into lymphatic node tissue. (Histology and immunohistochemistry Figure 9F, G, H is courtesy of Dr. Antoniuk S.A., Research Associate, Dr. Petrenko L.I., Junior Scientific Researcher, Dr. Burtyn O.V., National Cancer Institute, Kyiv, Ukraine)
FIGURE 9G. Trephine biopsy histology (F) shows, that in fragment of the lymph node tissue with presence of fibrous tissue, the growth of poorly differentiated malignant tumor (arrows) of epithelial nature with extensive necrosis in the tumor and lymph node tissue, hemorrhages is determined (hematoxylin and eosin; magnification x200). Immunohistochemistry (G, H) shows positive membrane reaction (arrows) with Cytokeratin AE1/AE3 and Cytokeratin 5/6. Reaction with CD45, S100 is negative (reaction with CD45- is determined in intact cells of the lymph node tissue). Thus, there is metastasis of poorly differentiated squamous cell carcinoma G3 into lymphatic node tissue. (Histology and immunohistochemistry Figure 9F, G, H is courtesy of Dr. Antoniuk S.A., Research Associate, Dr. Petrenko L.I., Junior Scientific Researcher, Dr. Burtyn O.V., National Cancer Institute, Kyiv, Ukraine)
FIGURE 9H. Trephine biopsy histology (F) shows, that in fragment of the lymph node tissue with presence of fibrous tissue, the growth of poorly differentiated malignant tumor (arrows) of epithelial nature with extensive necrosis in the tumor and lymph node tissue, hemorrhages is determined (hematoxylin and eosin; magnification x200). Immunohistochemistry (G, H) shows positive membrane reaction (arrows) with Cytokeratin AE1/AE3 and Cytokeratin 5/6. Reaction with CD45, S100 is negative (reaction with CD45- is determined in intact cells of the lymph node tissue). Thus, there is metastasis of poorly differentiated squamous cell carcinoma G3 into lymphatic node tissue. (Histology and immunohistochemistry Figure 9F, G, H is courtesy of Dr. Antoniuk S.A., Research Associate, Dr. Petrenko L.I., Junior Scientific Researcher, Dr. Burtyn O.V., National Cancer Institute, Kyiv, Ukraine)
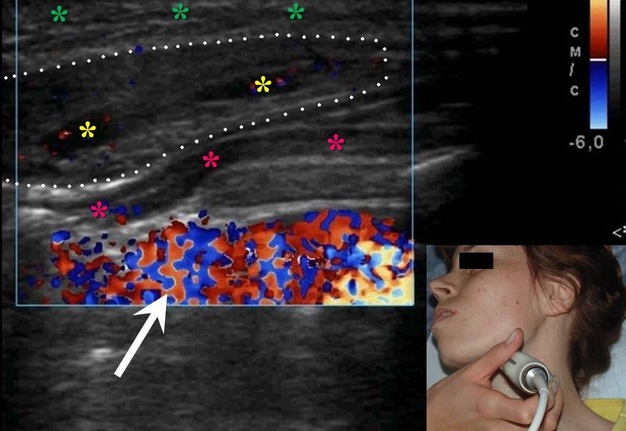
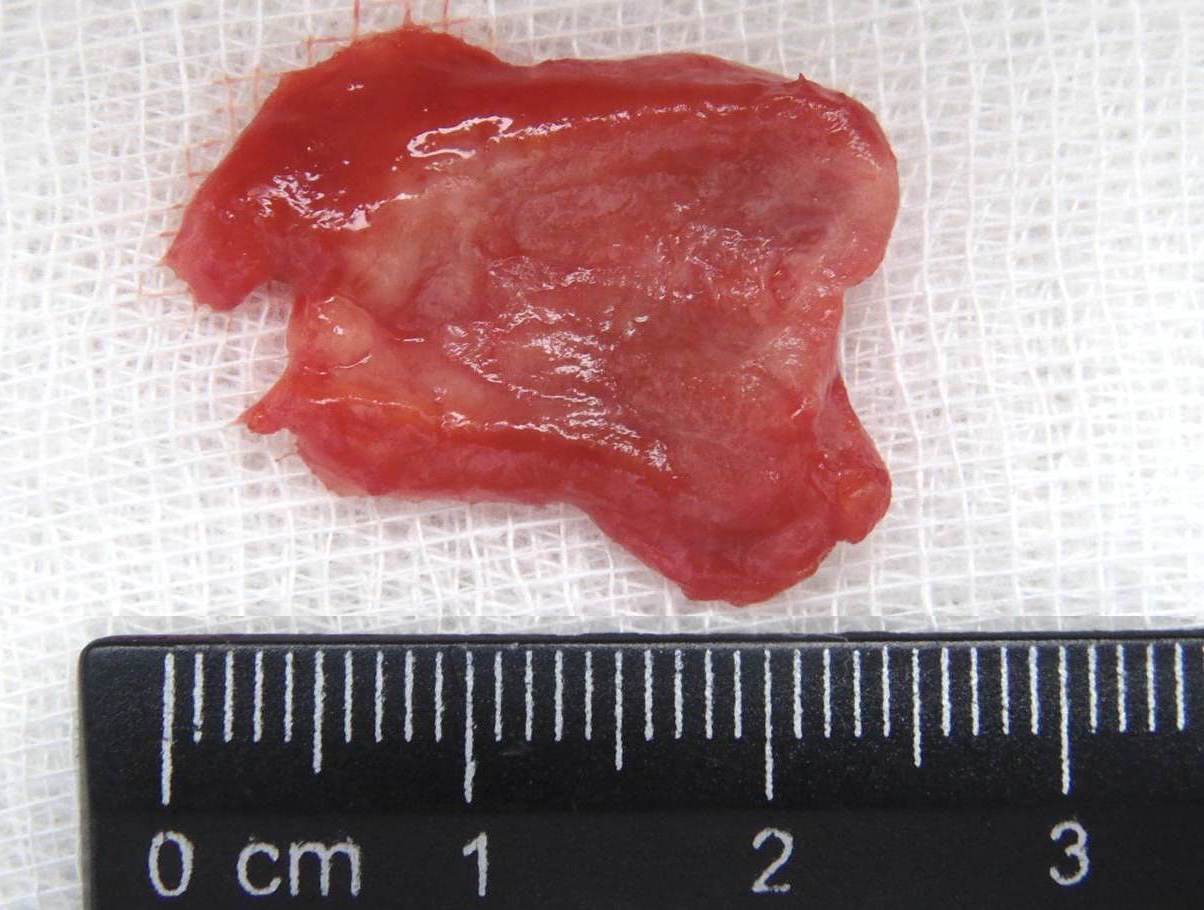
FIGURE 10A. A 32-years-old female with cavernous lymphangioma of the upper neck. Oblique color Doppler ultrasound (A) shows well-demarcated lesion (marked by white dots) with a size of 3.0- × 1.0- cm elongated-oval form at the upper neck adjust to the omohyoid (pink asterisks) and anterior to the sternocleidomastoid muscles. Structure of the lesion is heterogeneous with presence of oval anechoic areas (cavities − yellow asterisks). The skin, subcutaneous tissue is marked by green stars. White arrow marks blooming artifact. Lesion have no arterial or venous blood flow. Oblique color Doppler ultrasound (B) with the compression by transducer (sonopalpation) noted the tumor shrinkage in 2-3 times, cavernous cameras are completely disappeared, indicating its spongy structure and confirmed after removal (lesion for the entire thickness was impregnated with light-gray liquid − lymph). Surgical specimen (C). Histology (D). In the lumen of the lymph vessels, lymph (arrows) is visualized. The inner layer is composed of endothelial cells of lymphatic vessels (oval form small inclusions with dark blue color). Hematoxylin and eosin; magnification x200.
FIGURE 10B. A 32-years-old female with cavernous lymphangioma of the upper neck. Oblique color Doppler ultrasound (A) shows well-demarcated lesion (marked by white dots) with a size of 3.0- × 1.0- cm elongated-oval form at the upper neck adjust to the omohyoid (pink asterisks) and anterior to the sternocleidomastoid muscles. Structure of the lesion is heterogeneous with presence of oval anechoic areas (cavities − yellow asterisks). The skin, subcutaneous tissue is marked by green stars. White arrow marks blooming artifact. Lesion have no arterial or venous blood flow. Oblique color Doppler ultrasound (B) with the compression by transducer (sonopalpation) noted the tumor shrinkage in 2-3 times, cavernous cameras are completely disappeared, indicating its spongy structure and confirmed after removal (lesion for the entire thickness was impregnated with light-gray liquid − lymph). Surgical specimen (C). Histology (D). In the lumen of the lymph vessels, lymph (arrows) is visualized. The inner layer is composed of endothelial cells of lymphatic vessels (oval form small inclusions with dark blue color). Hematoxylin and eosin; magnification x200.
FIGURE 10C. A 32-years-old female with cavernous lymphangioma of the upper neck. Oblique color Doppler ultrasound (A) shows well-demarcated lesion (marked by white dots) with a size of 3.0- × 1.0- cm elongated-oval form at the upper neck adjust to the omohyoid (pink asterisks) and anterior to the sternocleidomastoid muscles. Structure of the lesion is heterogeneous with presence of oval anechoic areas (cavities − yellow asterisks). The skin, subcutaneous tissue is marked by green stars. White arrow marks blooming artifact. Lesion have no arterial or venous blood flow. Oblique color Doppler ultrasound (B) with the compression by transducer (sonopalpation) noted the tumor shrinkage in 2-3 times, cavernous cameras are completely disappeared, indicating its spongy structure and confirmed after removal (lesion for the entire thickness was impregnated with light-gray liquid − lymph). Surgical specimen (C). Histology (D). In the lumen of the lymph vessels, lymph (arrows) is visualized. The inner layer is composed of endothelial cells of lymphatic vessels (oval form small inclusions with dark blue color). Hematoxylin and eosin; magnification x200.
FIGURE 10D. A 32-years-old female with cavernous lymphangioma of the upper neck. Oblique color Doppler ultrasound (A) shows well-demarcated lesion (marked by white dots) with a size of 3.0- × 1.0- cm elongated-oval form at the upper neck adjust to the omohyoid (pink asterisks) and anterior to the sternocleidomastoid muscles. Structure of the lesion is heterogeneous with presence of oval anechoic areas (cavities − yellow asterisks). The skin, subcutaneous tissue is marked by green stars. White arrow marks blooming artifact. Lesion have no arterial or venous blood flow. Oblique color Doppler ultrasound (B) with the compression by transducer (sonopalpation) noted the tumor shrinkage in 2-3 times, cavernous cameras are completely disappeared, indicating its spongy structure and confirmed after removal (lesion for the entire thickness was impregnated with light-gray liquid − lymph). Surgical specimen (C). Histology (D). In the lumen of the lymph vessels, lymph (arrows) is visualized. The inner layer is composed of endothelial cells of lymphatic vessels (oval form small inclusions with dark blue color). Hematoxylin and eosin; magnification x200.
TREATMENT
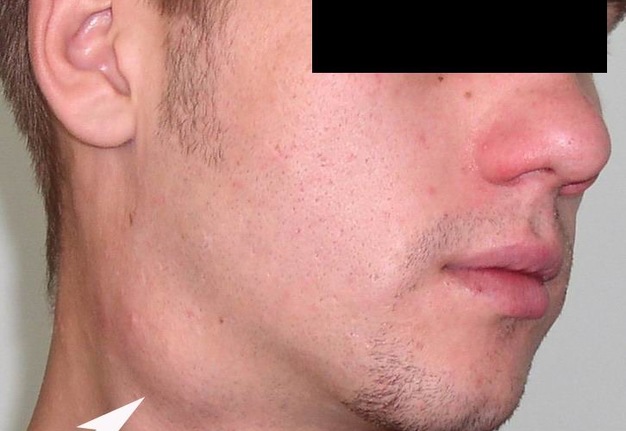
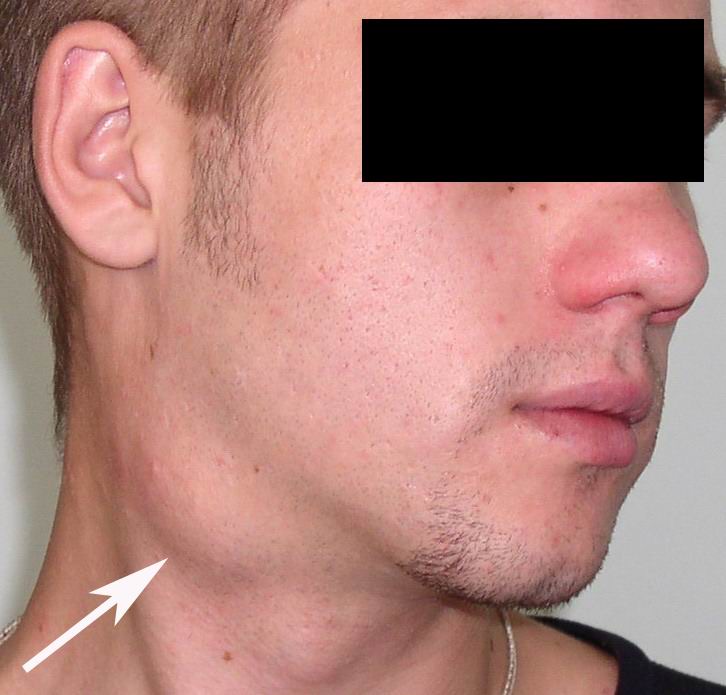
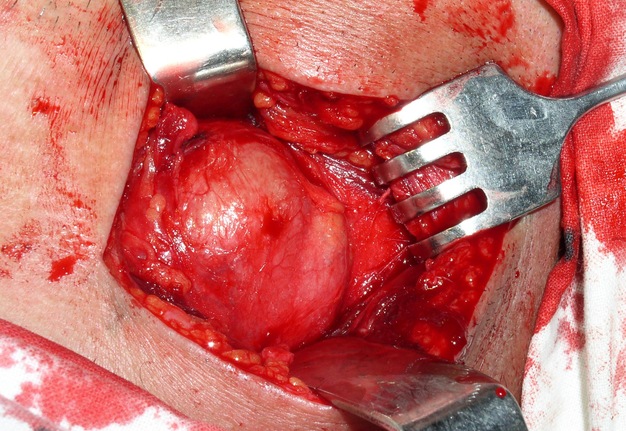
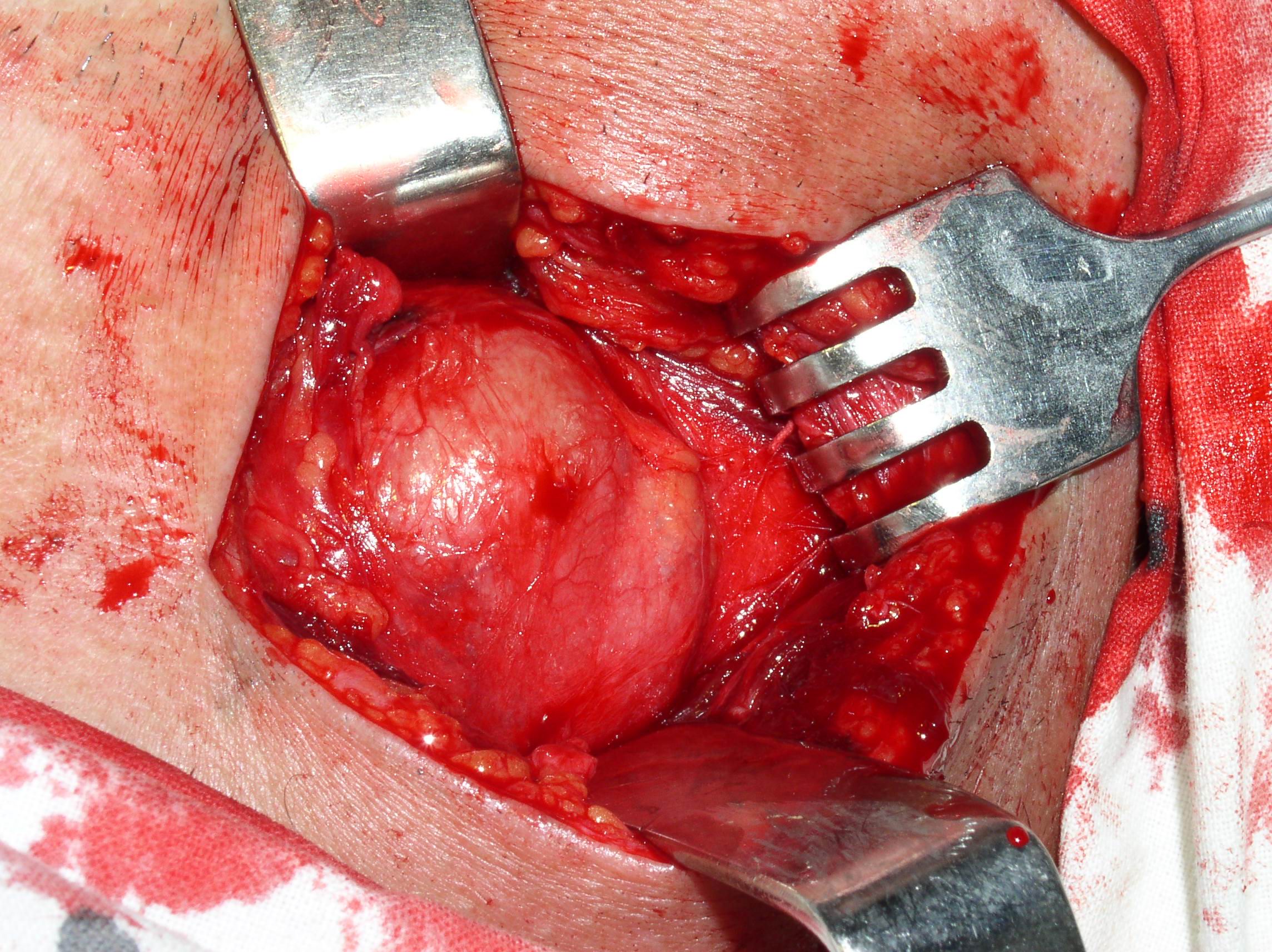
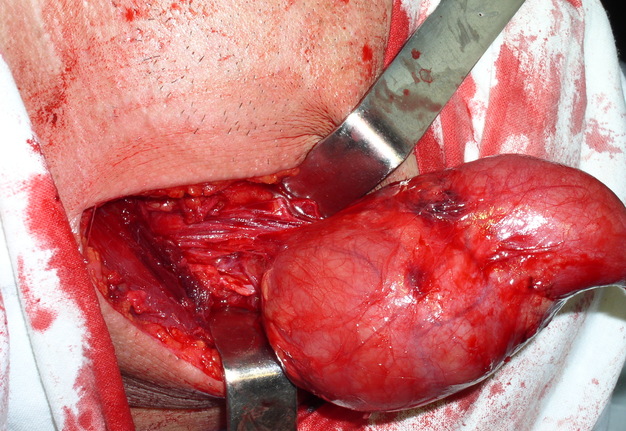
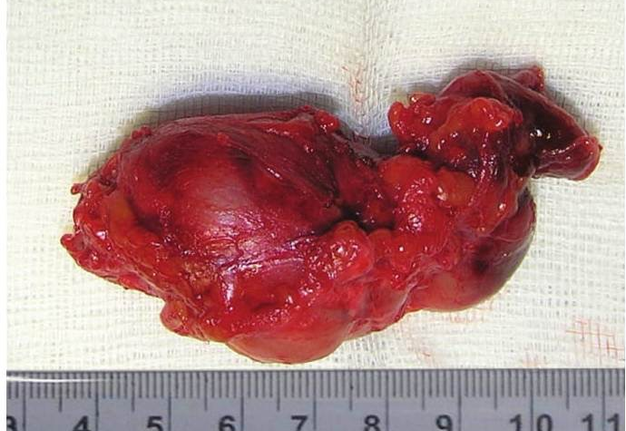
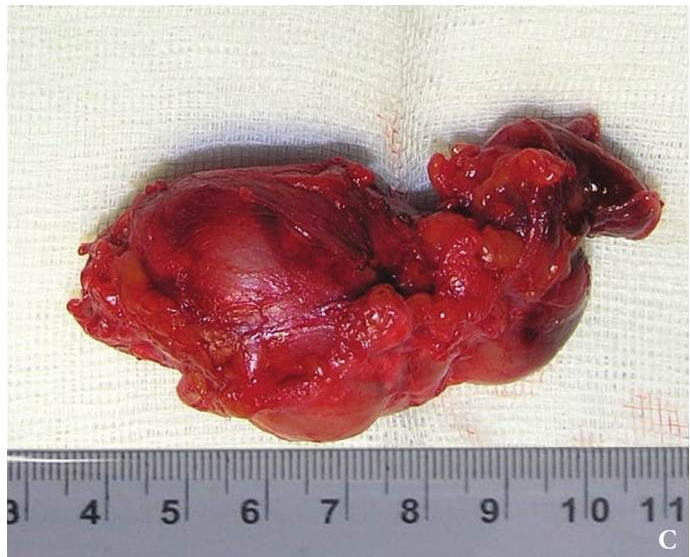
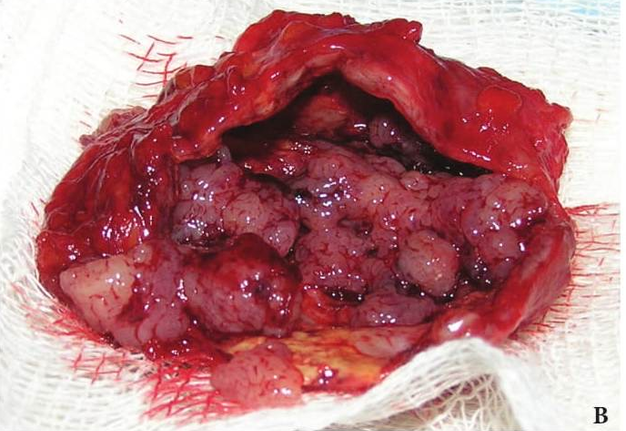
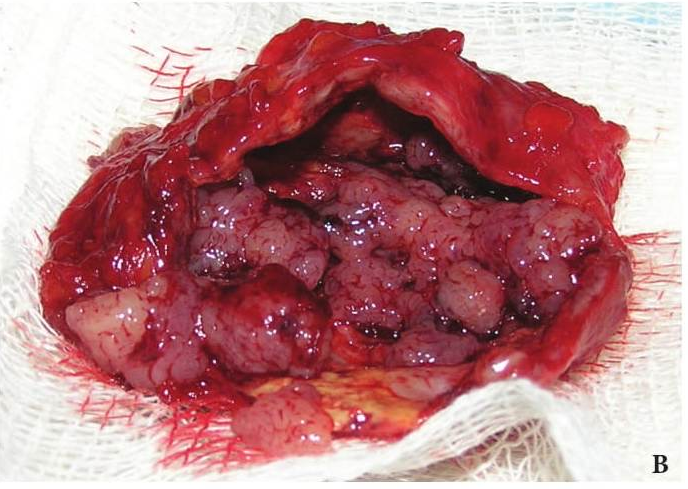
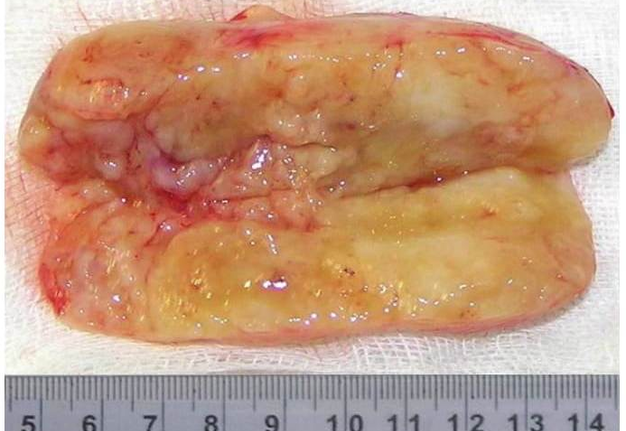
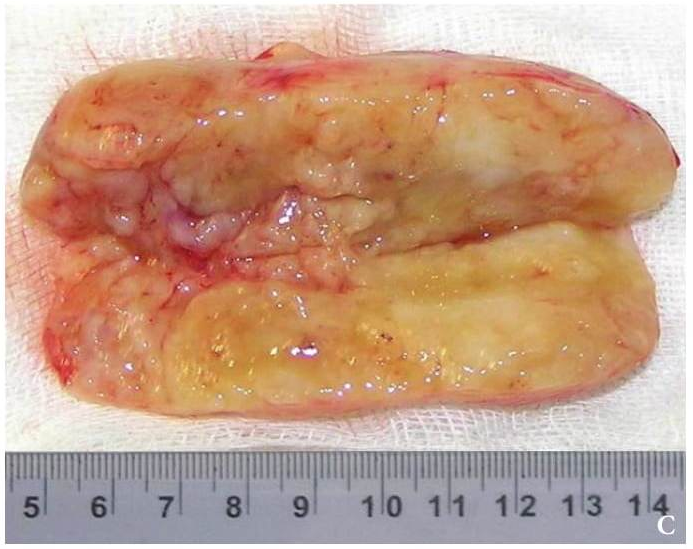
The treatment of the BCCs is only surgical (Figs 11, 12A). Surgery can be a difficult task due to the complex anatomical and topographical relations of the cysts with vessels and nerves of the neck. The surgery is performed under endotracheal anesthesia. The cut should be done on the anterior (medial) edge of the sternocleidomastoid muscle, or upper cervical crease. The first variant of incision is considered safer because in this area a large vessels and veins are located, and the second variant of cut − more cosmetic.
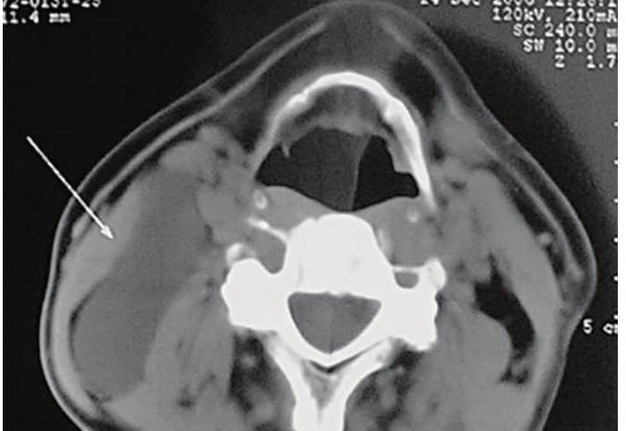
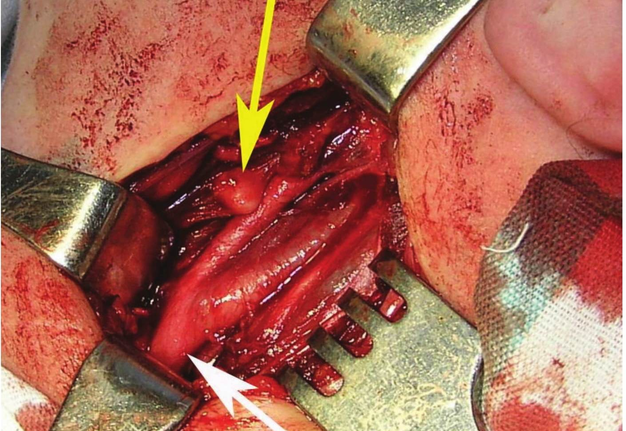
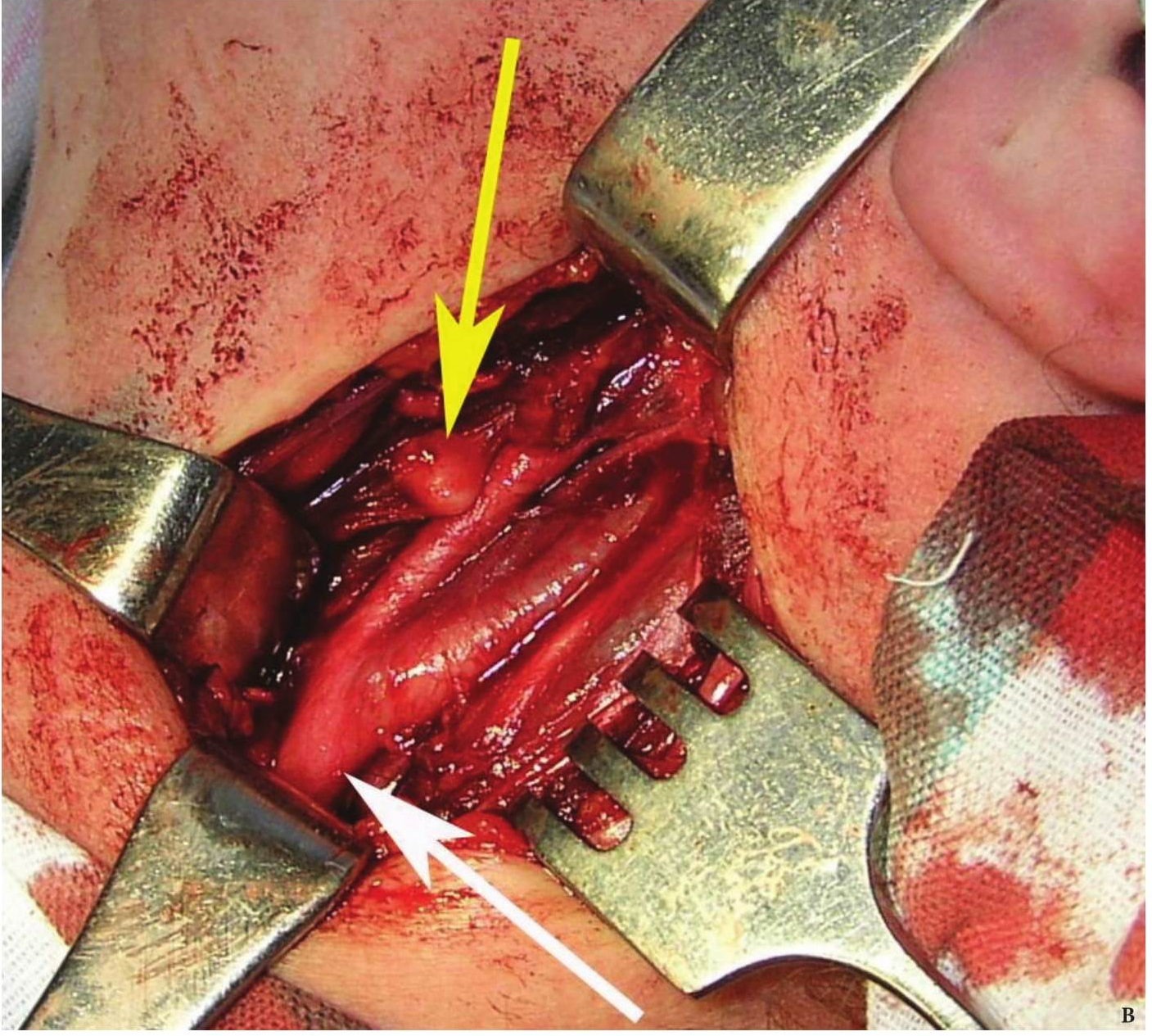
The surgeon may have difficulties in location place of internal pole of the BCC (Fig 1B), as in this place the internal jugular and facial veins are located. Especially need to be careful upon separating the BCC when its located nearby external and internal carotid arteries (Figs 12, 13). With the classical location of BCCs for the surgeon is more easily to navigate in the topographic anatomy of those vessels. However, often there are different variants for the location of the internal and external carotid arteries. This causes considerable difficulties in the intraoperative visualization of those vessels. Need to be especially careful in case of deep location of BCCs and the need to separate carotid arteries, i.e. topographic anatomy of the past is not always classical (Fig 13).
COMPLICATIONS
Among complications of BCCs are neck abscess and branchiogenic carcinoma. Phlegmon of the neck is more severe with severe intoxication in patient. Inflammatory processes can easily spread through the neurovascular bundle into the anterior mediastinum. Non-radical surgery may not only lead to recurrence, but also to the development of branchiogenic carcinoma (Figs 14, 15).
Branchiogenic carcinoma develops from the epithelium of the BCCs. Unlike cysts, tumor represents as a dense, tuberous, bad-movable (especially in the vertical direction) lesion, knitted with muscle and vascular bundle of the neck. Tumor (branchiogenic carcinoma) painless, relatively slow increases in size and can reach of considerable size, quickly merges to the surrounding tissues. Tumor localization: from the submandibular region to the clavicle. Branchiogenic carcinoma merges to sternocleidomastoid muscle and vascular bundle of the neck. If the tumor has not merges into the vascular bundle, it can be separated from the vessel. Malignant tumors can merges not only in vascular bundle neck, soft tissue (muscles) of the side of the neck, but to the pharynx and larynx. Histologically branchiogenic carcinoma usually has a structure of squamous cell carcinoma (Fig 9F, G, H).
Development of branchiogenic carcinoma, according to the Maxillofacial Surgery Department of Shupyk NMAPE is about 4.5% of patients with BCCs. A high percentage of branchiogenic cancer in these patients emphasizes the need for early and proper performed surgery (removal of the BCC). Treatment of patients with branchial cancer is combined. Prognosis is poor, often recurs, metastases are rare.
Branchiogenic carcinoma should be differentiated from the carotid chemodectoma (Fig 16) and other tumors of the neck. Carotid chemodectoma synonyms: carotid body paraganglioma, glomus tumor, endothelioma, perithelioma, pheochromocytoma, paraganglioma, potato tumor, receptoma, etc. Chemodectoma [34, 35] develops from the carotid sinus (synonyms: chemoreceptoral glomus, glomus caroticum), located at the adventitia layer inwards from the bifurcation of the common carotid artery.
Carotid sinus (Greek, karóō to put to sleep and sinus) is an expanded portion of the common carotid artery at the site of its branching into the external and internal arteries.
In this glomus there is a cluster of chromaffin cells around capillary glomeruli, and there is lot amount of nerve endings (functions as a "chemoreceptor" − responds to changes in the level of oxygen in the blood). Making pressure on the vessel in the area of carotid glomus leads to a slowing of heart rate. The carotid sinus baroreceptors are also located, when they are stimulated the blood vessels dilates and blood pressure falls. Chemodectomas are located under the sternocleidomastoid muscle at the point of common carotid artery bifurcation.
The skin over the tumor is not changed. Tumor has spherical or elongated form, with dimensions of 3.0 cm or more, smooth or slightly tuberous. A characteristic feature of chemodectoma is its horizontal displaceability and absence of displacement in vertical direction, the inability to move aside tumor from the pulsating vessel and "transfer pulsation" over the tumor.
Macroscopically carotid chemodectoma has a light gray or brownish-red color and is surrounded by a connective tissue capsule (Fig 16C). Treatment of chemodectoma is surgical. Postoperative mortality is very high, i.e. in the vast majority it is not possible to separate the tumor from the common or internal carotid artery.
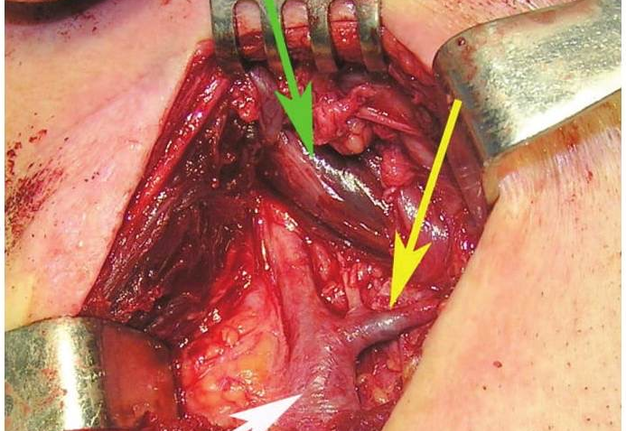
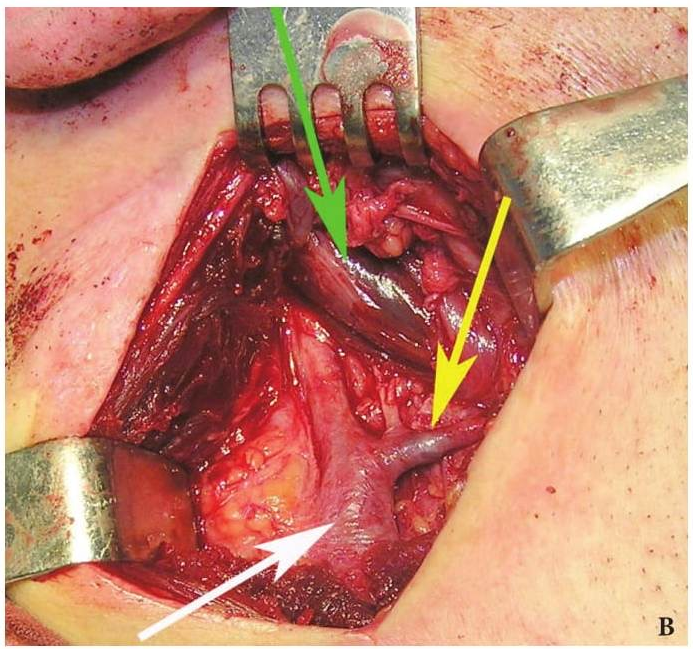
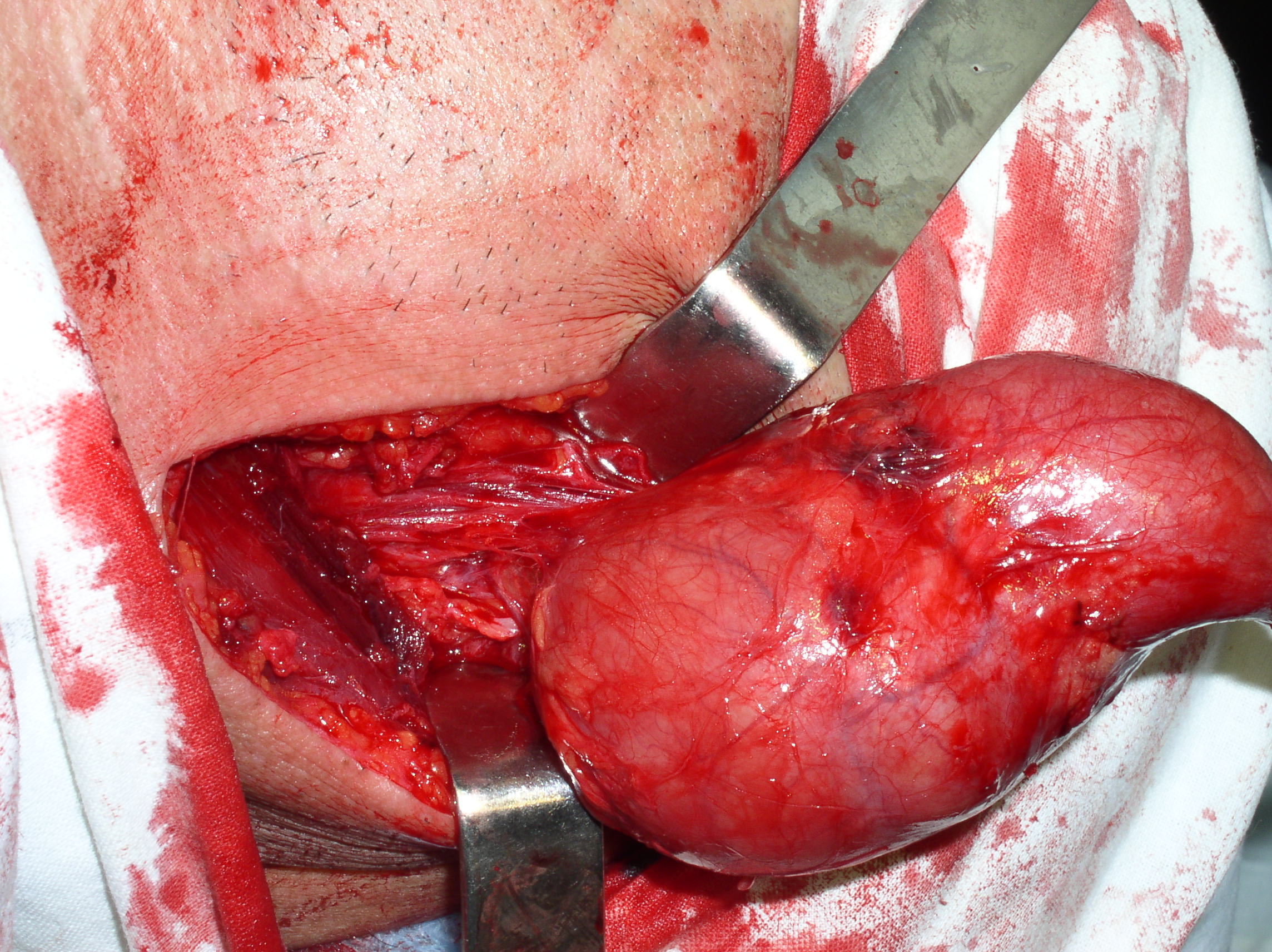
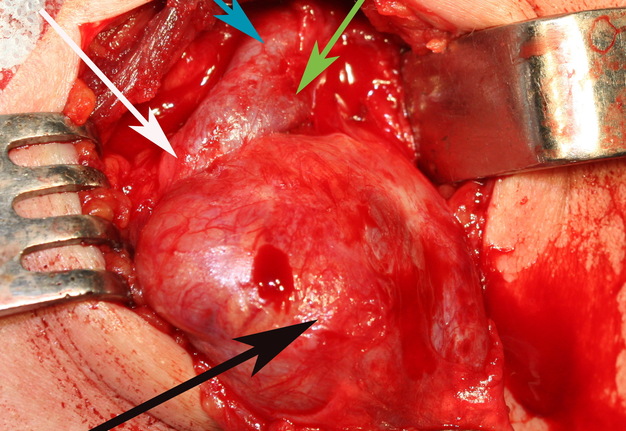
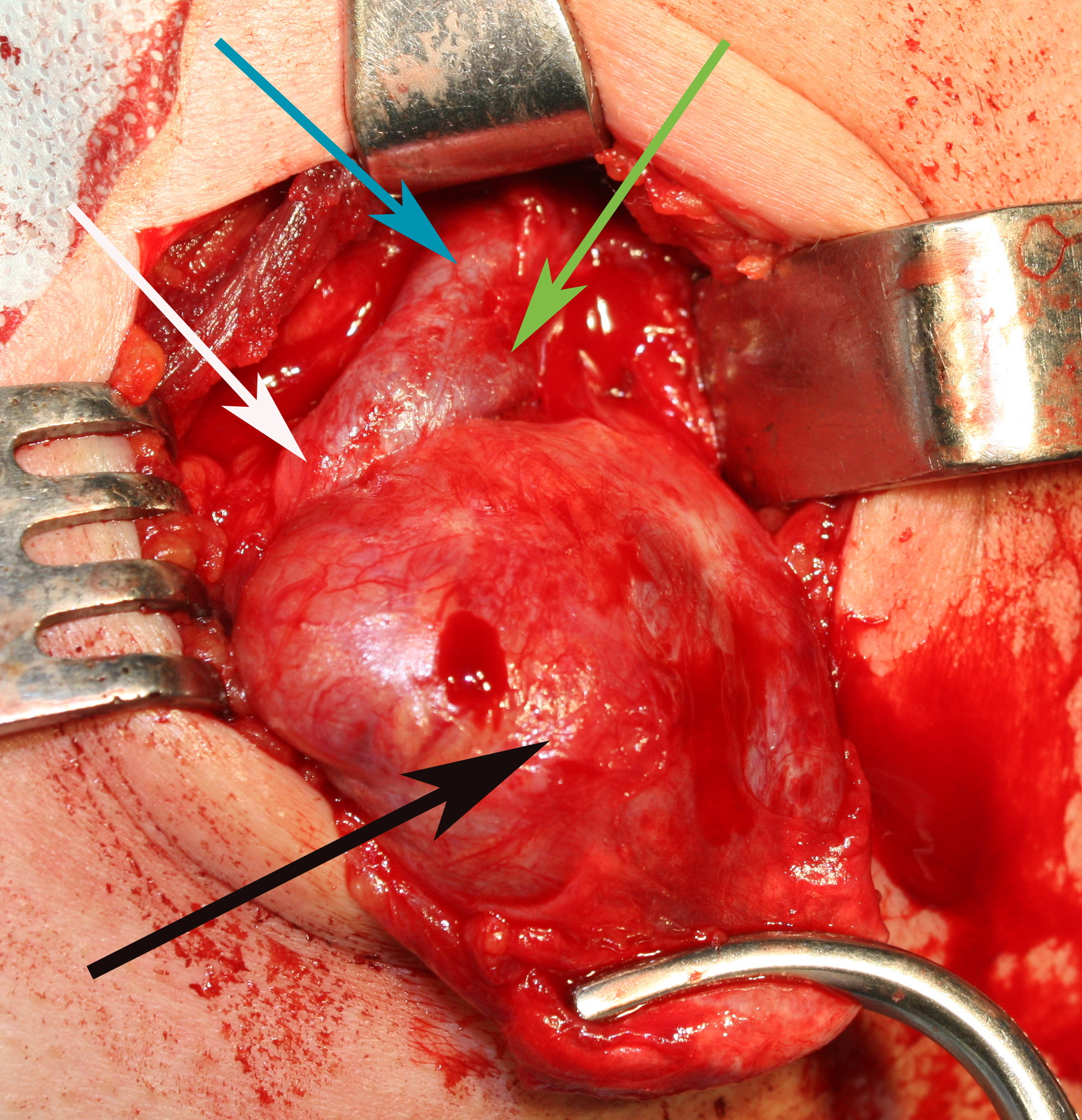
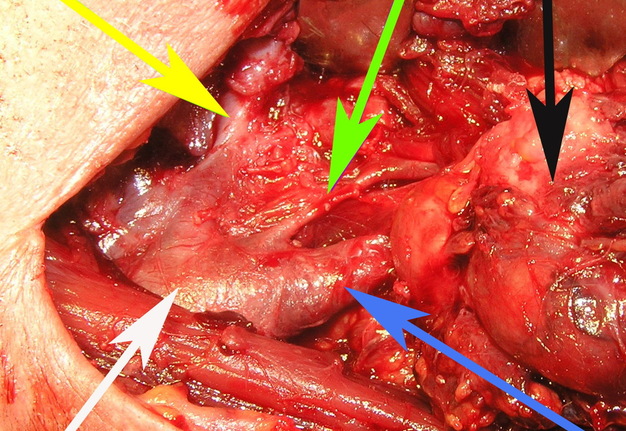
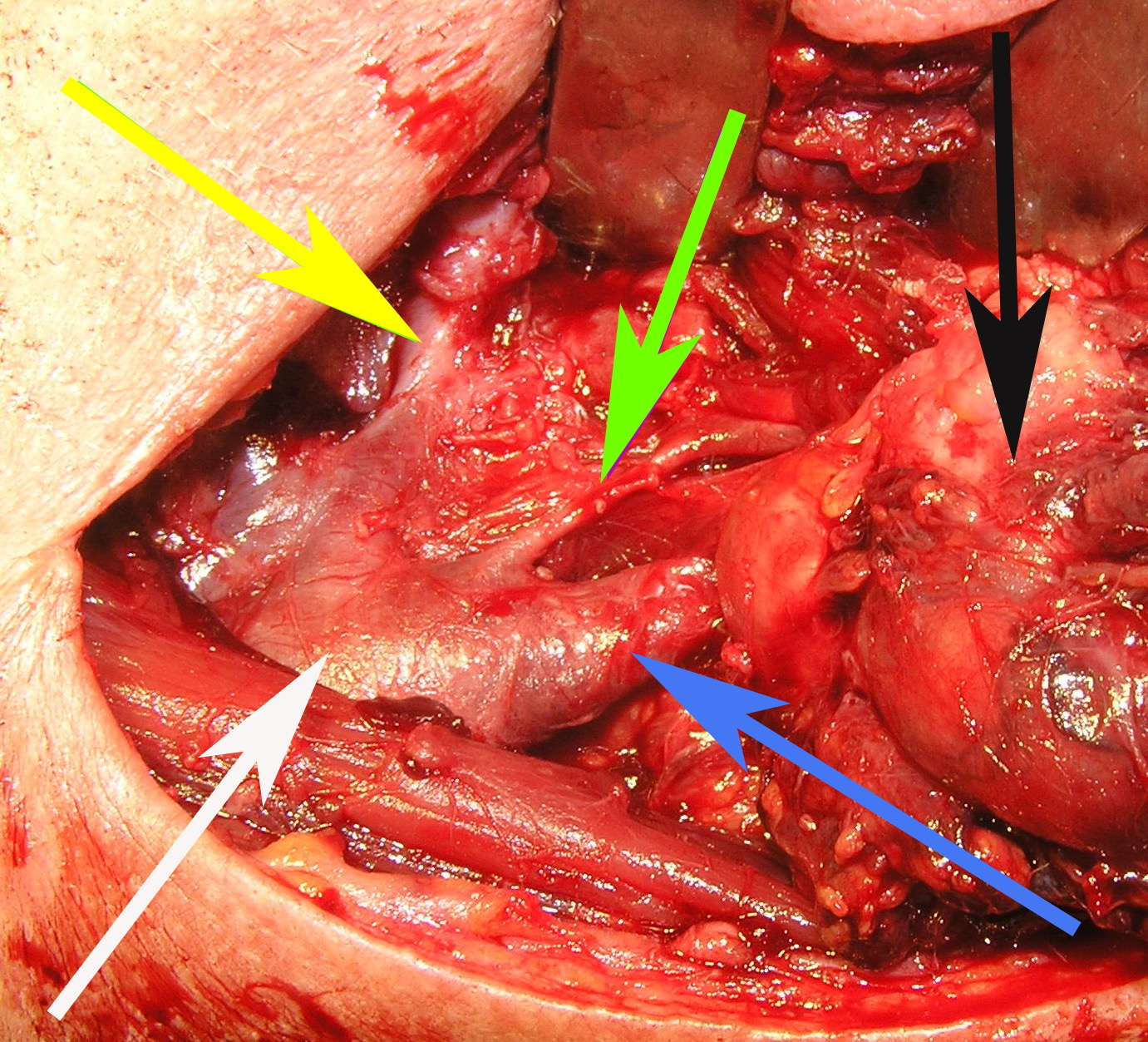
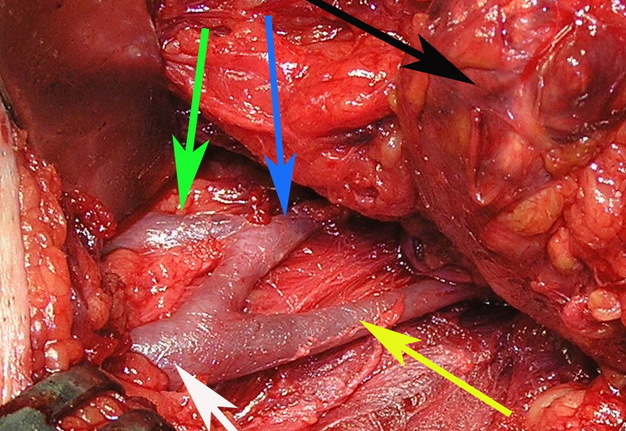
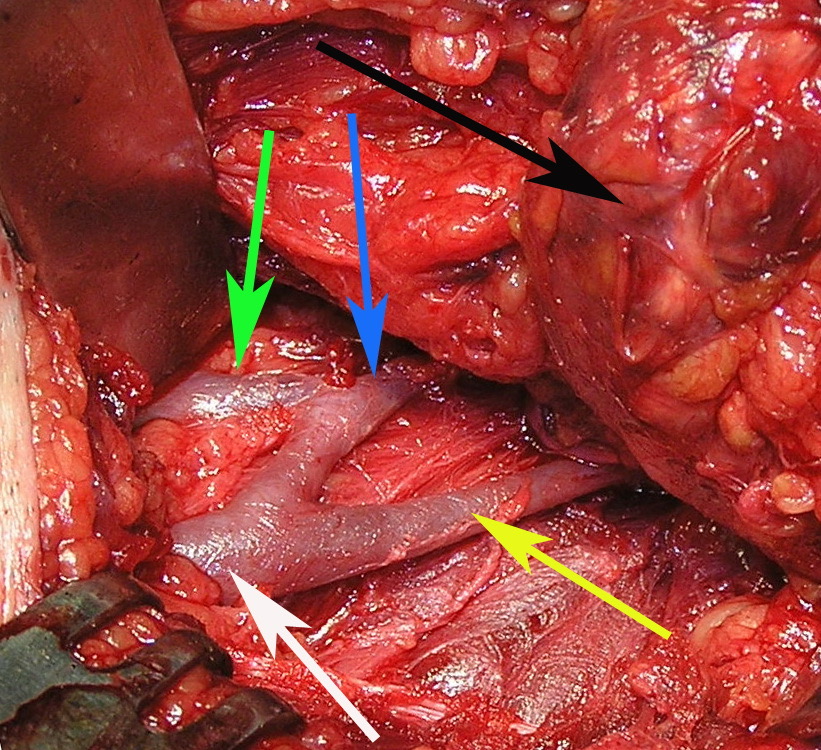
FIGURE 12A. A stage of the BCC removing (A). Black arrow − BCC; white arrow − common carotid artery; blue arrow − external carotid artery; green arrow − internal carotid artery. View of the surgical wound after the BCC excision (B). White arrow − common carotid artery; blue arrow − external carotid artery; green arrow − internal carotid artery.
FIGURE 12B. A stage of the BCC removing (A). Black arrow − BCC; white arrow − common carotid artery; blue arrow − external carotid artery; green arrow − internal carotid artery. View of the surgical wound after the BCC excision (B). White arrow − common carotid artery; blue arrow − external carotid artery; green arrow − internal carotid artery.
PROGNOSIS
With timely and correct performed surgery, removing of the BCCs, the prognosis is favorable.
References (35)
-
Tymofieiev AA. Rukovodstvo po chelustno-litsevoi hirurgii i hirurgicheskoi stomatologii [Manual of maxillofacial and oral surgery]. 5th ed. Kyiv: Chervona Ruta-Turs; 2012. p. 741−6 (in Russian).
-
Timofieiev AA. Chelustno-litsevaia hirurgiia [Maxillofacial surgery]. 2nd ed. Kyiv: Meditsina; 2015. p. 498−501 (in Russian).
-
Tymofieiev OO. Schelepno-lytseva hirurgiia [Maxillofacial surgery]. 1st ed. Kyiv: Meditsyna; 2011. p. 473−5 (in Ukrainian).
-
Kotliarov PM, Kharchenko VP, Aleksandrov YK, et al. Ultrazvukovaia diagnostika zabolevanii schitovidnoi zhelezy [Diagnostics ultrasound of the thyroid gland diseases]. 2nd ed. Vidar; 2009. p. 171, 172 (in Russian).
-
Lanham PD, Wushensky C. Second brachial cleft cyst mimic: case report. AJNR Am J Neuroradiol 2005;26:1862–4.
-
Chen PS, Lin YC, Lin YS. Nasopharyngeal branchial cleft cyst. Journal of the Chinese Medical Association 2012;75:660e662.
-
Chavan S, Deshmukh R, Karande P,et al. Branchial cleft cyst: A case report and review of literature. J Oral Maxillofac Pathol 2014;18:150.
-
Hu S, Hu CH, Yang L, et al. Atypical imaging observations of branchial cleft cysts. Oncol Lett 2014;7:219–22.
-
Bajaj Y, Tweedie D, Ifeacho S, et al. Surgical technique for excision of first branchial cleft anomalies: how we do it. Clin Otolaryngol 2011; 36:371–4.
-
Chan KC, Chao WC, Wu CM. Surgical management of first branchial cleft anomaly presenting as infected retroauricular mass using a microscopic dissection technique. Am J Otolaryngol 2012;33:20.
-
Guo YX, Guo CB. Relation between a first branchial cleft anomaly and the facial nerve. Br J Oral Maxillofac Surg 2012;50: 259–63.
-
Pradipta KP, Arun A, Kalairasi R, et al. First branchial cleft malformation with duplication of external auditory canal. Case Reports in Otolaryngology 2013;2013:1−5.
-
Krishnamurthy A, Ramshanker VA. Type I first branchial cleft cyst masquerading as a parotid tumor. Natl J Maxillofac Surg 2014;5:84–85.
-
Joshi MJ, Provenzano MJ, Smith RJ, et al. The rare third branchial cleft cyst. AJNR Am J Neuroradiol 2009;30:1804−6.
-
Adams A, Mankad K, Offiah C, et al. Branchial cleft anomalies: a pictorial review of embryological development and spectrum of imaging findings. Insights Imaging 2016;7:69–76.
-
Bailey H. Branchial cysts and other essays on surgical subjects in the faciocervical region. London: Lewis,1929.
-
Wenig BM. Atlas of head and neck pathology. 3rd ed. Elsevier; 2015. p. 538−46.
-
Mittal MK, Malik A, Sureka B, et al. Cystic masses of neck: a pictorial review. Indian J Radiol Imaging 2012;22:334−43.
-
Ahuja A, Ying M. Sonographic evaluation of cervical lymphadenopathy: is power Doppler sonography routinely indicated? Ultrasound Med Biol 2003;29:353–9.
-
Zenk J, Bozzato A, Steinhart H, et al. Metastatic and inflammatory cervical lymph nodes as analyzed by contrast-enhanced color-coded Doppler ultrasonography: quantitative dynamic perfusion patterns and histopathologic correlation. Ann Otol Rhinol Laryngol 2005;114:43–7.
-
Hsieh YY, Hsueh S, Hsueh Ch, et al. Pathological Analysis of Congenital Cervical Cysts in Children: 20 Years of Experience at Chang Gung Memorial Hospital. Chang Gung Med J 2003;26:107−13.
-
Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg 2005;132:938−42.
-
Dutta M, Saha J, Biswas G, et al. Epidermoid Cysts in Head and Neck: Our Experiences, with Review of Literature. Indian J Otolaryngol Head Neck Surg 2013;65(Suppl 1):14–21.
-
Amer I, Choudhury N, Falzon A, et al. Cystic neck masses – a diagnostic and management challenge. SAJ Cas Rep 2014;1:1−4.
-
Kraus J, Plzák J, Bruschini R, et al. Cystic lymphangioma of the neck in adults: a report of three cases. Wien Klin Wochenschr. 2008;120:242−5.
-
Karkos PD, Spencer MG, Lee M, et al. Cervical cystic hygroma/lymphangioma: an acquired idiopathic late presentation. J Laryngol Otol 2005;119:561–3.
-
Kennedy TL, Whitaker M, Pellitteri P, et al. Cystic hygroma/lymphangioma: a rational approach to management. Laryngoscope 2001;111:1929–37.
-
Grasso DL, Pelizzo G, Zocconi E, et al. Lymphangiomas of head and neck in children Acta Otorhinolaryngol Ital 2008;28:17–20.
-
Dutta M, Kundu S, Ghosh B. Cystic squamous cell carcinoma of the neck: сould a second metastatic focus help? Otolaryngologia Polska 2014;68:338−41.
-
Ahuja AT, King AD, Metreweli C. Second branchial cleft cysts: variability of sonographic appearances in adult cases. AJNR Am J Neuroradiol 2010;21:315–9.
-
Yuen HY, Ahuja AT. Chapter 11: benign clinical conditions in the adjacent neck. In: Sofferman RA, Ahuja AT, editors. Ultrasound of the thyroid and parathyroid glands. Springer; 2012. p. 229−33.
-
Caly DN, Viana A, Rapoport A, et al. Indications and pitfalls of immunohistochemistry in head and neck cancer. Braz J Otorhinolaryngol 2013;79:75−81.
-
Naswa N, Kumar A, Sharma P, et al. Imaging carotid body chemodectomas with 68Ga-DOTA-NOC PET-CT. Br J Radiol 2012;85(1016):1140–5.
-
Davidovic LB, Djukic VB, Vasic DM, et al. Diagnosis and treatment of carotid body paraganglioma: 21 years of experience at a clinical center of Serbia. World J Surg Oncol 2005; 3:10.
-
Durdik S, Malinovsky P. Chemodectoma − carotid body tumor surgical treatment. Bratisl Lek Listy 2002;103:422−3.