A Case Report of Oral Mucosa Metastasis of Renal Clear Cell Carcinoma Mimicking Benign Neoplasm: Possibilities and Limitations of the Ultrasonography
July 31, 2025
J Diagn Treat Oral Maxillofac Pathol 2025; 9: 100307.
DOI: 10.23999/j.dtomp.2025.7.100307
Under a Creative Commons license (CC BY-NC-SA 4.0)
HOW TO CITE THIS ARTICLE
Cherniak OS, Demidov VH, Petrychenko O, Blyzniuk VP. A case report of oral mucosa metastasis of renal clear cell carcinoma mimicking benign neoplasm: Possibilities and limitations of the ultrasonography. J Diagn Treat Oral Maxillofac Pathol. 2025 Jul;9(7):100307. https://doi.org/10.23999/j.dtomp.2025.7.100307
NATIONAL REPOSITORY OF ACADEMIC TEXTS
https://nrat.ukrintei.ua/en/searchdoc/2025U000052/
ABSTRACT
Oral metastases are rare, accounting for approximately 1-1.5% of all oral malignancies. Therefore, it is important for clinicians to create diagnostic data for early verification of this formidable pathological process. The most common primary tumors that metastasise to the oral cavity are: lung (21.1%), liver (12.3%), breast (10.5%), kidney (10.5%) and colorectal (8.8%). We hope that the publication of case described below will complement the international literature and bring ultrasound diagnostics to a new level. A 49-year-old Caucasian female with a growing neoplasm in the oral cavity and its mushroom-shaped attachment to the mucosa of the mandibular alveolar process presented for surgical examination.The neoplasm clinically mimicked a benign pathology. The anterior part of the neoplasm was examined using ultrasonography with the presentation of grayscale and color Doppler sonograms. It is worth noting that the vestibular (anterior, smaller) part of the metastasis in this case did not have a characteristic sign for metastases in other parts of the body, namely, there was no vascularization on color Doppler ultrasonography. The article also provides clinical and orthopantomography data. The limitations of the linear probe for a complete ultrasound assessment of the neoplasms located on the lingual or possibly palatal sides of the alveolar processes were analyzed. A comparative analysis of a linear and a hockey-stick probes is presented. After conducting our analysis of the literature, we can state that this article presents for the first time data on ultrasound examination of oral mucosa metastasis of renal clear cell carcinoma. Further investigation of each oral metastasis using intraoral direct ultrasound examination is extremely important for systematizing the ultrasound variants of this pathology. In turn, this will allow clinicians to more likely establish a correct preliminary diagnosis before biopsy and treat this pathology in specialized departments.
|
Takeaways
Question: Is there a solution for ultrasound imaging and diagnosis of neoplasms located on the lingual and palatal side of the alveolar processes?
Findings: Considering that the use of direct ultrasonography from the lingual and palatal sides of the alveolar ridge is difficult to access with a linear probe, we suggest using a linear hockey stick probe. This is especially important in the early diagnosis of oncological processes. Meaning: Providing the possibility of direct contact of the hockey-stick linear probe with pathological tissues will allow for their full ultrasound examination while preserving grayscale, color, and power Doppler sonograms. |
INTRODUCTION
Metastatic tumors to the oro-facial region are uncommon and account for approximately 1-1.5% of all malignant oral tumors [1, 2]. The most common primary tumors that metastasise to the oral cavity are: lung (21.1%), liver (12.3%), breast (10.5%), kidney (10.5%) and colorectal (8.8%) [3]. That is, out of 5 different groups of organs from which cancer can metastasize to the oral cavity, kidney cancer ranks 4th in frequency. Renal cell carcinoma (RCC) is the most common type of primary renal cancer, and clear-cell is the most common subtype [4]. The ultrasonography (USG) patterns of malignant lymph nodes are well described [5]. Although there are quite a few published cases of renal cell carcinoma metastasis to the oral cavity [2, 6-15], we were unable to find a single case with documented USG of such metastases. This may be due to the rarity of the pathology itself, the absence of USG in the examination protocol, and the lack of a special ultrasound probe for insertion into the oral cavity.
So, the purpose of this report is to highlight the case of oral mucosa metastasis of renal clear cell carcinoma, present the results of first to our knowledge ever reported ultrasound examination of such pathologic condition.
CASE REPORT
In May 19, 2015, a 49-year-old Caucasian female was referred to the Center of Maxillofacial Surgery and Dentistry with complaints of the presence of a neoplasm on the mucosa of the alveolar process of the mandible. According to the patient, this neoplasm has existed for the past few months and is growing. When taking the history, the patient did not mention any surgeries or treatments for RCC.
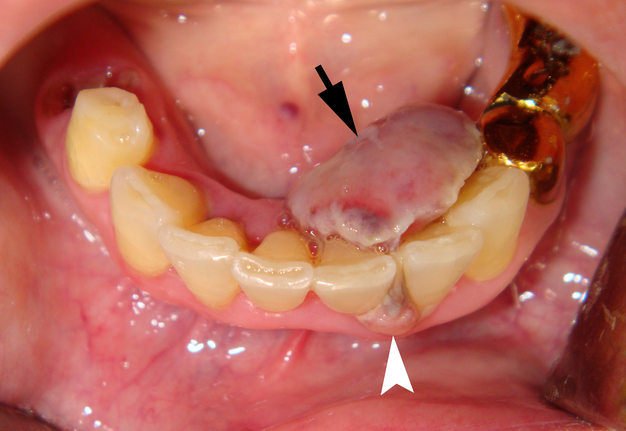
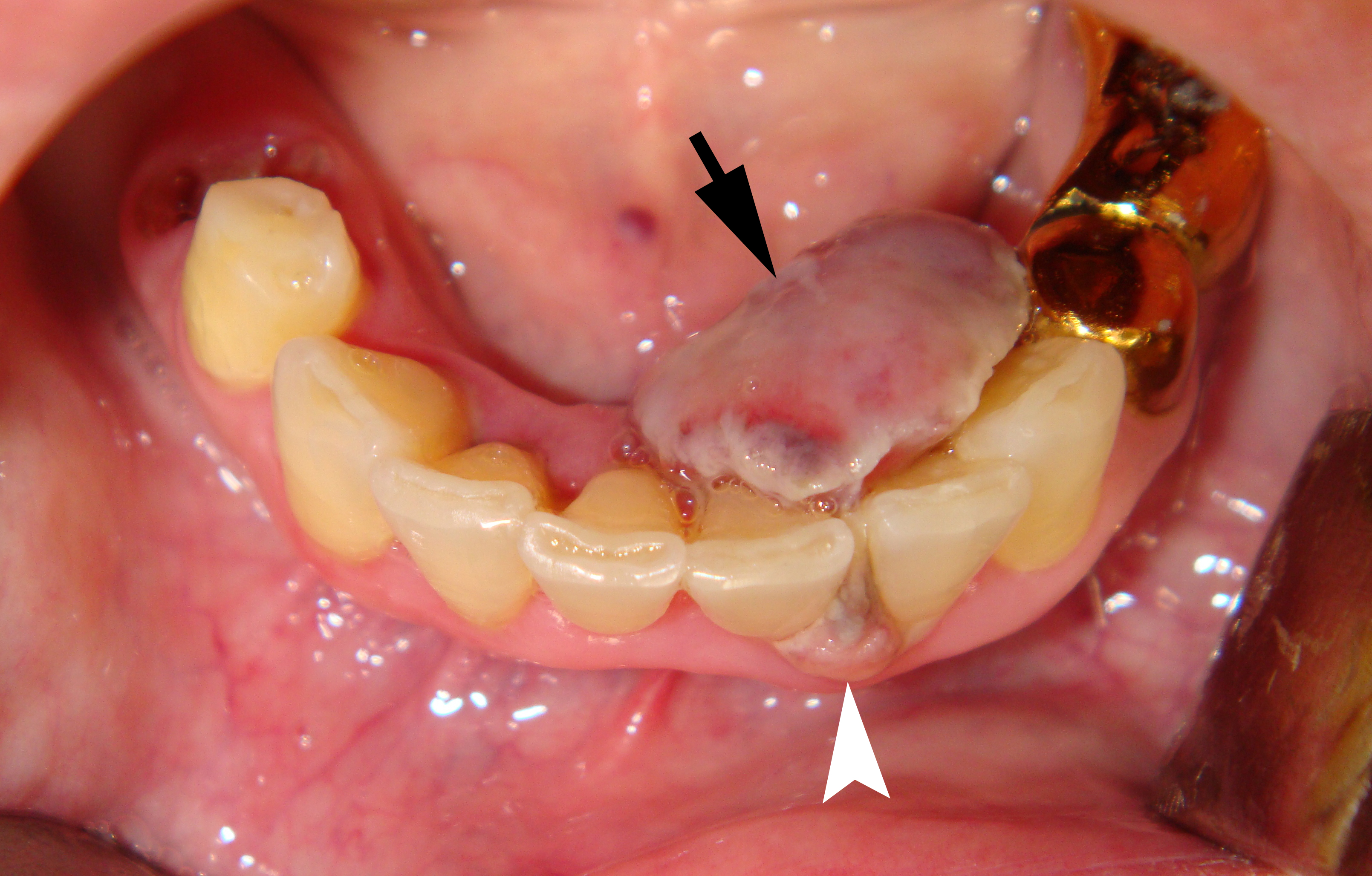
Clinical examination revealed the presence of a neoplasm on the lingual side of the alveolar process of the mandible that had grown vestibularly between the left central and lateral incisors (Fig 1). The neoplasm was mushroom-shaped, pale pink in color, elastic upon palpation, and had a pedicle. The mucosa adjacent to the neoplasm is unchanged in color and structure. The neoplasm measured 1.8 x 1.1 x 1.2 cm at the lingual aspect of the lower teeth and 0.34 x 0.38 cm at the vestibular aspect.
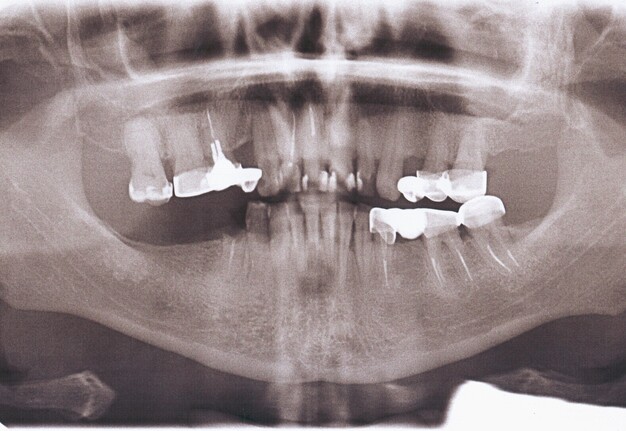
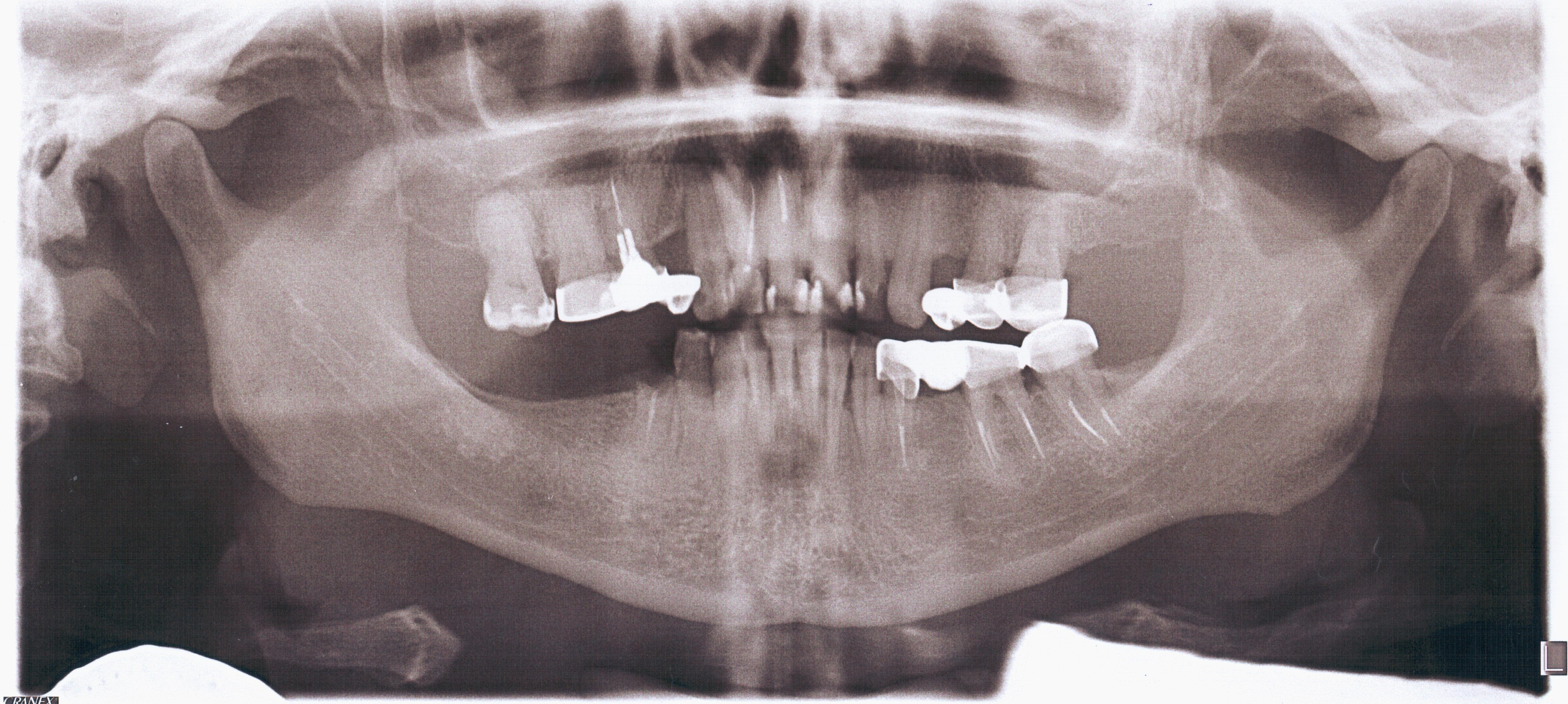
Orthopantomography (Fig 2) showed a minor area of alveolar ridge resorption in the area between the lower left lateral incisor and canine.
An extraoral ultrasound examination was performed by O.S.C., an expert physician (>18 years of experience in head and neck USG), on the day of admission in the department. A HD11 XE USG system (2007, the Philips, Amsterdam, the Netherlands) equipped with a linear L12-3 transducer (frequency fange: 3.0-12.0 MHz; aperture: 38 mm) was used for the examination, fixing a 3-cm of ultrasonography (penetration) depth.
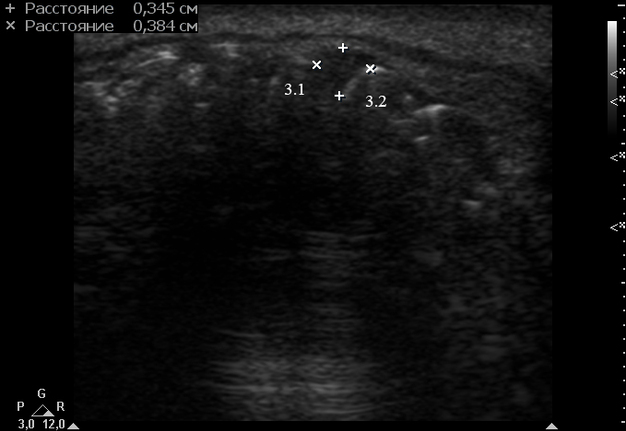
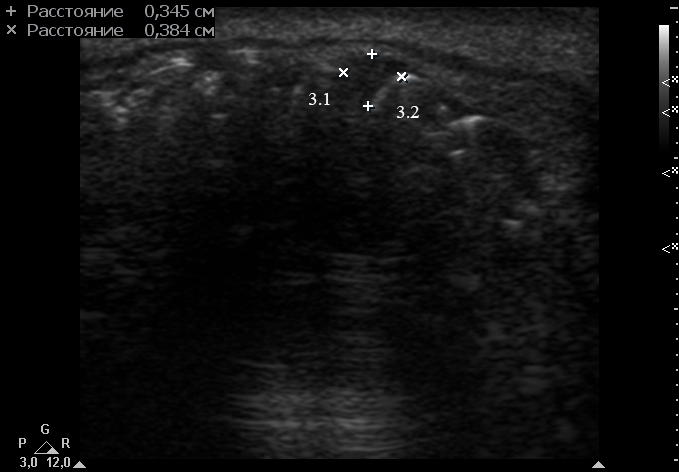
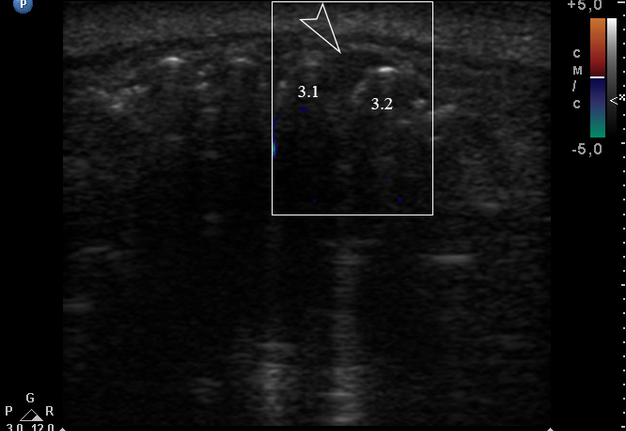
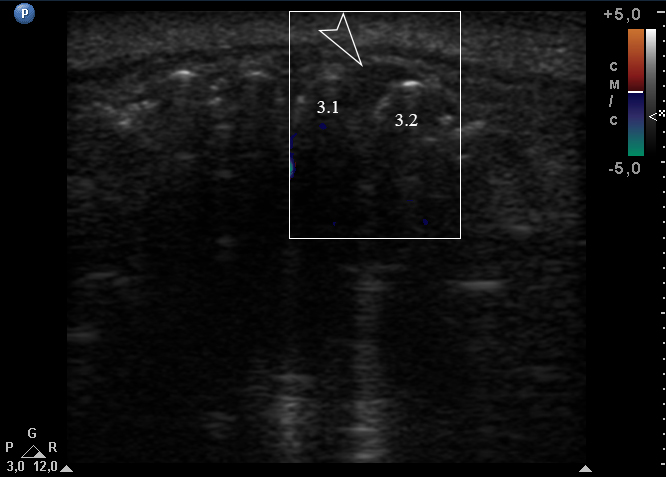
Anterior part of the neoplasm at the vestibular aspect of the lower teeth measured 0.34 x 0.38 cm (Fig 3). Upon the gray-scale USG the anterior part portion of the neoplasm vizualized as homogenous hypoechoic lesion with no signs of calcifications. Color Doppler USG showed no vascularity within the vestibular portion of the neoplasm (Fig 4).
Unfortunately, this ultrasound machine in our hospital was equipped only with a linear and convex probe but was not equipped with a hockey-stick probe. Application of linear probe “hockey stick” is a solution for ultrasound imaging and diagnostics of neoplasms located on the lingual and palatal sides of the teeth, i.e., for the intraoral USG.
This ultrasound machine that we used to examine this patient, namely HD11 XE, is suitable for a transducer such as L15-7io hockey stick ultrasound probe (the Philips, Amsterdam, the Netherlands; frequency range: 7.0-15.0 MHz; aperture: 23 mm).
An excisional biopsy of the neoplasm was performed under general anesthesia. The histopathological diagnosis of metastasis of renal clear cell carcinoma was made by experienced pathologists. The patient was referred for further treatment to an oncologist.
DISCUSSION AND TECHNIQUE
Metastasis is a spread of cancer cells from the place where they first formed to another part of the body [16]. In metastasis, cancer cells break away from the original (primary) tumor, travel through the blood or lymph system, and form a new tumor in other organs or tissues of the body [16].
In metastatic nodes, upon the gray-scale USG assessment of size, shape, borders, echogenicity, echogenicity of the hilum, presence of intranodal necrosis, calcifications, and ancillary features (matting of the nodes and adjacent soft tissue oedema) is performed [5]. Upon the Doppler USG of the metastatic nodes vascular distribution and resistance are examining [5].
USG of skin metastasis (arms or buttock localization) of carcinomas is already presented in the literature [17-19]. Key characteristics according to Corominas et al (2015) were next: (1) metastasis of the prostate adenocarcinoma was iso/hypoechoic on gray scale sonograms, moderately well-defined, and was joined to a vessel that fed it, and (2) the marked vascularity on color/power Doppler [17].
USG pattern of metastasis according to Martínez-Morán et al (2018) was next: A heterogeneous solid-cystic lesion in the dermis and extending into the subcutaneous tissue [18]. The lesion also had anechoic central areas admixed with hyperechoic areas with well-defined borders [18]. It displayed posterior reinforcement and power Doppler USG showed peripheral vascular poles [18].
Bai et al (2022) reported the cutaneous metastasis of RCC [19]. Superficial USG of the right arm in their case showed (1) an oval, hypo-echoic nodule with well-defined margins and no shadowing in the subcutaneous fat, (2) extensive vascularity [19].
Pickhardt and Pickard (2003) reported the delayed thyroid metastasis from RCC with jugular vein extension and color Doppler showed prominent metastatic tumor vascularity [20].
Even though many sources describe the ultrasound picture of carcinoma metastases, USG patterns of the oral metastasis of the RCC are not described. In any case, we were unable to describe a single case with an ultrasound picture of oral metastasis of the RCC. It is important to know that renal clear cell carcinoma is also terms as hypernephroma, Grawitz tumor, and renal cell carcinoma (Makos and Psomaderis [2009]) [7]. Metastasis of the renal clear cell carcinoma spread to different organs including bones, the brain, the pancreas, the gallbladder, lymph nodes [21], and oral cavity [2, 6-15].
In the case of benign neoplasm/primary malignant tumor/metastatic tumor in the oral cavity, clinicians can visually assess their size, shape, surface, and structure with and without palpation. In addition to the clinical examination, USG will allow to assess the internal structure, the boundaries of the neoplasm, the depth of invasion into adjacent tissues and vascularization. Some cases of USG in the intraoral benign and malignant tumors are well described by Orhan and Ünsal (2021) [22].
Again, we reviewed a significant number of sources describing oral metastases of RCC and found no ultrasound images. In particular, van der Waal et al (2003) in their report on 24 cases of oral metastases indicate that in four cases the site of the primary tumor was the kidney, and the site of oral metastases was the maxilla, mandible, soft palate, and buccal mucosa [2]. Histological type in these 4 cases was clear cell carcinoma [2]. The age of these patients ranged from 48 to 67 years, and the sex ratio was 50/50% [2].
Hirshberg et al (2008) performed important analyzis of 673 intraoral metastasis of carcinomas from different primary sites [23]. The jawbones (n = 36) were more frequently affected by metastasis of RCC than the oral soft tissues (n = 29). For example, Kelles et al (2012) presented a 59-year-old woman who had left nephrectomy because of renal cell carcinoma three years ago referred with trismus and a metastasis on her temporomandibular joint [24].
We consider the following cases noteworthy, as they allow for clinical comparison with our case. Sastre et al (2005) described a 64-year-old male with a metastasis along the right alveolar ridge of the mandible [6].
Makos and Psomaderis (2009) published a case of a 63-year-old male with a maxillary epulis-like metastasis from the right central maxillary incisor to the first right maxillary premolar [7].
Dashow et al (2011) highlighted a case of 67-year-old gentleman metastatic clear cell renal carcinoma of the palate mimicking noninvoluting congenital hemangioma [8]. Metastasis visualized as anonhealing, exophytic lesion on his soft-hard palate, which the patient had been aware of for approximately one year. The patient had a history of a renal cell carcinoma diagnosis in 1991, which had been treated with radical right nephrectomy.
Milner et al (2014) reported a 67-year-old male with a metastasis on the hard palate mucosa which looked somewhat like a case of Dashow et al (2011) [8, 9].
Selvi et al (2016) presented three synchronous atypical metastases of RCC to the maxillary gingiva, scalp and the distal phalanx of the fifth digit in a 51-year-old male [10].
Vasilyeva et al (2018) described a metastasis of RCC as a tan-red exophytic, lobulated mass of the maxillary anterior facial gingiva with a separate, similar appearing smaller lesion identified in the right maxillary vestibule [25].
Nisi et al (2020) reported two males, 61- and 71-year-old, with oral metastases located on the left tongue and buccal mucosa [11].
Kovalski et al (2020) detailed double lobe nodule covering the gingiva and alveolar ridge on the left side of the maxilla in a 63-year-old male [12]. The metastasis measured 5 x 3 cm, had a reddish colouration, was covered by a purulent membrane, bled easily when touched and had a foul odour.
Patel et al (2020) delineate oral metastasis in a 59-year-old female as a pink-red, oval, ulcerated lesion with a white pseudomembranous surface measuring approximately 3.8 x 2.5 x 1.7 cm attached to the left buccal mucosa via a pedunculated stalk [14].
Intraoral metastasis of RCC is also described as exophytic tumor mass, sized about 6 x 3 cm. In the anterioposterior direction, it extended from the mandible level, involving the alveolar portion, to the retromolar triangle on the right; craniocaudally, the lesion extended from the lower fornix, over the top of the alveolar process, crossing it over, and descending toward the floor of the mouth (Stojanovic et al, 2020). In the described case in a 53-year-old male was also noted a enlargement of the right half of the face [13].
Khushbu et al (2024) reported metastasis to the upper gingiva in a 52-year-old female [26].
Metastasis to the upper maxilla in a 79-year-old male from the study by Nakako et al (2025) was clinically similar to our case [15].
Interestingly, dermatologic USG requires using multifrequency linear or compact linear (hockey-stick) probes [27]. Probes working with their upper-frequency range between 15 and 24 MHz allow displaying the skin layers and their abnormalities with sufficiently high-quality images [27]. Ultrasound in skin cancer and skin metastases are ilustrated in the study by Wortsman (2024) [28].
Healthcare professionals may attempt to examine various areas of the oral cavity both extra- and intraoral USG.
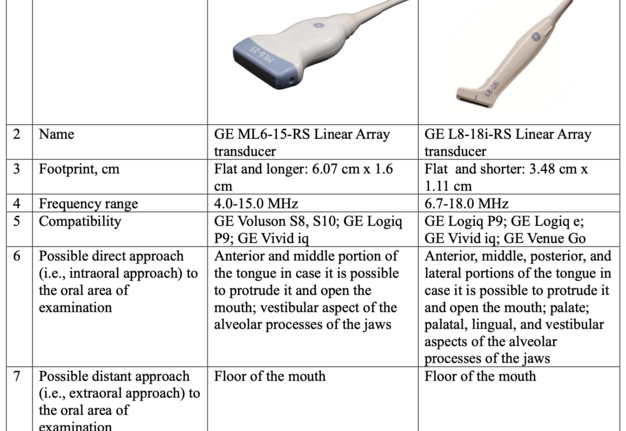
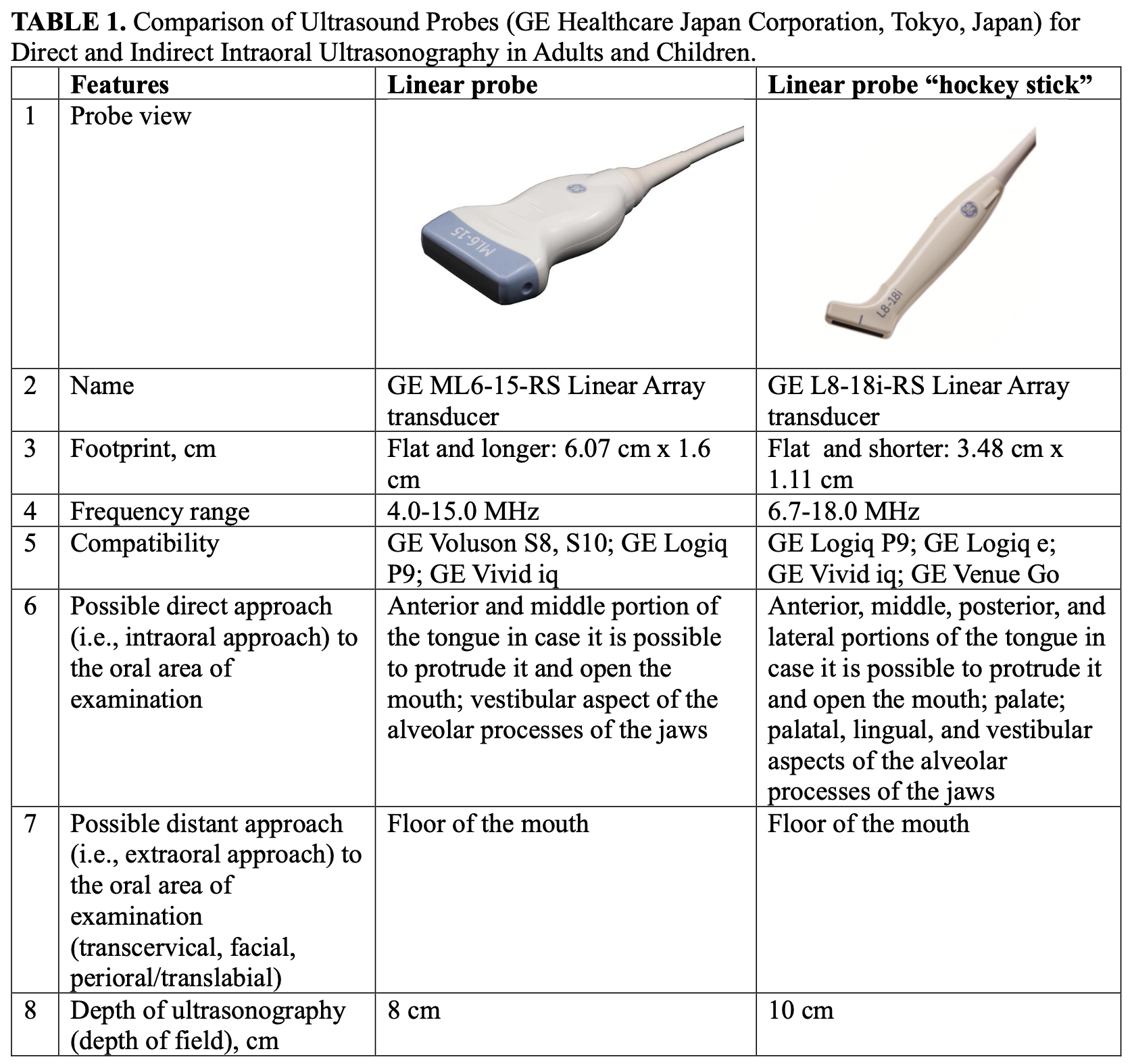
We consider it advisable and indication to use a hockey stick probe for intraoral ultrasound scanning of all intraoral lesions. A comparison of the characteristics of linear and hockey-stick probes from one manufacturer, namely GE (GE Healthcare Japan Corporation, Tokyo, Japan), is presented in Table 1.
The use of “hockey stick” probe is also very usefull for young and small patients due to the lack of space for the use of the regular linear probe (Guimarães Ferreira Fonseca et al, 2024) [29]. Synonymous name for the linear probe “hockey stick” is a small-footprint linear array transducer.
The use of a linear transducer for intraoral ultrasound scanning and diagnosis of pathology that is in close proximity to the transducer surface is possible. For example, this is possible for diagnosing tongue pathology, provided there is sufficient mouth opening and/or some tongue mobility [30-33]. However, the use of a hockey-type transducer is the most rational, in particular for cases like ours.
The lower level of floor of mouth and the lower margin of the mandible can be assessed perfectly with extraoral ultrasound using a linear transducer [34-36]. Ultrasonographic capabilities in examining the floor of the mouth and the root of the tongue using linear and curved probes are presented in the study by Abbott et al (2020) [32]. The clinicians should understand that penetration depth is the highest distance between the probe and the tissues which can still be visualized without image noise [37].
Thus, our proposal is to use a hockey-stick probe [38, 39] in cases with a pathology location like our case. As an option, we suggest using the following transducer: Linear probe “hockey stick” GE L8-18i-RS (GE Healthcare Japan Corporation, Tokyo, Japan).
One of the important points in obtaining high-quality informative sonograms when using a linear probe is the proximity of the target diagnostic area (area with a pathological focus) to the surface of the probe [40]. Since with a remote localization of the pathology area, the diagnostic ability decreases due to the likelihood of the presence of anatomical objects/foreign bodies that can produce artifacts and due to the technical limitations of the ultrasound probe itself. That is, ideally, the area with pathology should be located at a depth of 0 to 3.0-6.0 cm relative to the footstep of the transducer.
According to the literature (Cheng et al, 2000; Dashow et al, 2011), the 5-year survival rate for RCC after nephrectomy is 60% to 75%; with a solitary oral metastasis, the treatment is radical excision and nephrectomy, with a 5-year survival rate of 35% [41, 8]. The prognosis for patients with multiple RCC metastases is poor, with a 5-year survival rate of 0% to 7%.
In summary, metastasis to the oral cavity is rare, accounting for approximately 1-1.5% of all oral malignancies [1, 42, 43]. Therefore, it is extremely important to develop diagnostic data for the verification of such metastases, including ultrasound data. It is worth noting that the vestibular (anterior, smaller) part of the metastasis in this case did not have a characteristic sign for metastases in other parts of the body, namely, there was no vascularization on color Doppler ultrasonography. Hypothetically, this can be explained either by the initial stage of metastasis development [44] and the location in relation to soft tissues and bony structures.
CONCLUSION
Further investigation of each oral metastasis using intraoral direct ultrasound examination is extremely important for systematizing the ultrasound variants of this pathology. In turn, this will allow clinicians to more likely establish a correct preliminary diagnosis before biopsy and treat this pathology in specialized departments.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
PATIENT CONSENT
Informed consent was obtained from the patient at the time of presentation.
REFERENCES (44)
-
Kumar G, Manjunatha B. Metastatic tumors to the jaws and oral cavity. J Oral Maxillofac Pathol. 2013 Jan;17(1):71-5. https://doi.org/10.4103/0973-029X.110737
-
van der Waal RI, Buter J, van der Waal I. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. 2003 Feb;41(1):3-6. https://doi.org/10.1016/s0266-4356(02)00301-7
-
Liu Y, Vargo RJ, Bilodeau EA. Analytic survey of 57 cases of oral metastases. J Oral Pathol Med. 2018 Mer;47(3):275-280. https://doi.org/10.1111/jop.12672
-
Silver E, Roudakova K, Bial N, Daniel D. Cutaneous metastasis of renal cell carcinoma to the cheek: A case report and literature review. Am J Case Rep. 2021 Mar 26;22:e928999. https://doi.org/10.12659/AJCR.928999
-
Ahuja AT, Ying M, Ho SY, et al. Ultrasound of malignant cervical lymph nodes. Cancer Imaging. 2008 Mar 25;8(1):48-56. https://doi.org/10.1102/1470-7330.2008.0006
-
Sastre J, Naval L, Muñoz M, Gamallo C, Diaz FJ. Metastatic renal cell carcinoma to the mandible. Otolaryngol Head Neck Surg. 2005;132(4):663-664.
-
Makos CP, Psomaderis K. A literature review in renal carcinoma metastasis to the oral mucosa and a new report of an epulis-like metastasis. J Oral Maxillofac Surg. 2009;67(3):653-660. https://doi.org/10.1016/j.joms.2008.10.006
-
Dashow JE, Gemmete JJ, McHugh JB, Helman JI. Metastatic clear cell renal carcinoma of the palate mimicking noninvoluting congenital hemangioma. J Oral Maxillofac Surg. 2011;69(6):1836-1841. https://doi.org/10.1016/j.joms.2010.07.050
-
Milner P, Janas A, Grzesiak-Janas G. Clear cell renal carcinoma metastasis in the oral cavity – Case report. J Pre Clin Clin Res. 2014 Jun;8(2):127-129. https://doi.org/10.26444/jpccr/71484
-
Selvi F, Faquin WC, Michaelson MD, August M. Three synchronous atypical metastases of clear cell renal carcinoma to the maxillary gingiva, scalp and the distal phalanx of the fifth digit: A case report. J Oral Maxillofac Surg. 2016 Jun;74(6):1286.e1-1286.e12869. https://doi.org/10.1016/j.joms.2016.01.054
-
Nisi M, Izzetti R, Graziani F, Gabriele M. Renal cell carcinoma metastases to the oral cavity: Report of 2 cases and review of literature. J Oral Maxillofac Surg. 2020 Sep;78(9):1557-1571.https://doi.org/10.1016/j.joms.2020.04.001
-
Kovalski LNS, Ribeiro JT, Martins MD, et al. A rare case of oral metastasis of renal clear cell carcinoma: case report and review of literature. J Oral Diagn. 2020 Jan;5:1-9. https://doi.org/10.5935/2525-5711.20200006
-
Stojanovic M, Krasic D, Trajkovic M, Petrovic V. Rare renal cell carcinoma metastasis to mandibular gingiva: A case report and literature review. Niger J Clin Pract. 2020;23(10):1483-1486. https://doi.org/10.4103/njcp.njcp_55_19
-
Patel S, Barros J, Nwizu NN, Ogbureke KUE. Metastatic renal cell carcinoma to the oral cavity as first sign of disease: A case report. Clin Case Rep. 2020;8(8):1517-1521.
-
Nakako Y, Fujimoto T, Wada H, et al. Oral metastatic tumor (renal cell carcinoma) in maxillary gingiva: A case report and systematic review. J Oral Maxillofac Surg Med Pathol. 2025 Jul;37(4):839-848. https://doi.org/10.1016/j.ajoms.2025.01.004
-
National Cancer Institute: Metastasis [internet]. 2025 Jun 07 [cited 2025 Jun 07]. In Ukrainian. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/metastasis#
-
Corominas H, Estrada P, Reina D, Cerdà-Gabaroi D. Ultrasonography as a diagnostic tool for skin metastasis of a prostate adenocarcinoma. Reumatol Clin. 2016 Jan-Feb;12(1):54-56. https://doi.org/10.1016/j.reuma.2015.03.002
-
Martínez-Morán C, Echeverría-García B, Khedaoui R, Borbujo J. Cutaneous metastasis in a patient with lung cancer. Actas Dermosifiliogr (Engl Ed). 2018 May;109(4):372-374. https://doi.org/10.1016/j.ad.2017.05.019
-
Bai M, Li Z, He P. Ultrasound findings of subcutaneous soft tissue metastasis of renal cell carcinoma: A case report. Radiol Case Rep. 2022 Nov 1;18(1):192-195. https://doi.org/10.1016/j.radcr.2022.09.055
-
Pickhardt PJ, Pickard RH. Sonography of delayed thyroid metastasis from renal cell carcinoma with jugular vein extension. AJR Am J Roentgenol. 2003;181(1):272-274. https://doi.org/10.2214/ajr.181.1.1810272
-
Kanwal R. Metastasis in renal cell carcinoma: Biology and treatment. 2023 Jul;7:100094.https://doi.org/10.1016/j.adcanc.2023.100094
-
Orhan K, Ünsal G. Applications of ultrasonography in maxillofacial/intraoral benign and malignant tumors. In: Ultrasonography in dentomaxillofacial diagnostics. Orhan K, ed. Springer Nature Switzerland AG 2021; 275-318. https://doi.org/10.1007/978-3-030-62179-7_18
-
Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity - pathogenesis and analysis of 673 cases. Oral Oncol. 2008;44(8):743-752. https://doi.org/10.1016/j.oraloncology.2007.09.012
-
Kelles M, Akarcay M, Kizilay A, Samdanci E. Metastatic renal cell carcinoma to the condyle of the mandible. J Craniofac Surg. 2012 Jul;23(4):e302-e303. https://doi.org/10.1097/scs.0b013e318252f331
-
Vasilyeva D, Peters SM, Philipone EM, Yoon AJ. Renal cell carcinoma metastatic to the maxillary gingiva: A case report and review of the literature. J Oral Maxillofac Pathol. 2018 Jan;22(Suppl 1):S102-S107. https://doi.org/10.4103/jomfp.JOMFP_69_17
-
Krushbu, Navik, Sarthak, et al. Unusual metastasis of renal cell carcinoma to upper gingiva: A rare case report.Int J Life Sci Biotechnol Pharma Res. 2024 Feb;13(2):42-45.
-
Catalano O, Corvino A. Ultrasound of skin cancer: What we need to know. Semin Ultrasound CT MR. 2024;45(3):216-232. https://doi.org/10.1053/j.sult.2023.11.003
-
Wortsman X. Ultrasound in skin cancer: Why, how, and when to use it? Cancers. 2024; 16(19):3301. https://doi.org/10.3390/cancers16193301
-
Guimarães Ferreira Fonseca L, Bertolizio G, Engelhardt T, Karlsson J. Pediatric point of care airway ultrasound (POCUS): Current evidence and future practice. Anaesthesiologie. 2024 Jan 30. https://doi.org/10.1007/s00101-024-01377-6
-
Demidov VH, Cherniak OS, Snisarevskyi PP, et al. Schwannoma of the tongue: ultrasonography. J Diagn Treat Oral Maxillofac Pathol. 2022 Nov 30;6(11):138–47. https://doi.org/10.23999/j.dtomp.2022.11.2
-
Fesenko II. Abscess of the left tongue. J Diagn Treat Oral Maxillofac Pathol. 2020 Feb 28;4(2):39-40. https://doi.org/10.23999/j.dtomp.2020.2.5
-
Abbott S, Zelesco M, Gibson, D. Tongue sonography: An introduction. Sonography. 2020 Aug 03;7(3):118-125. https://doi.org/10.1002/sono.12230
-
Caprioli S, Casaleggio A, Tagliafico AS, et al. High-frequency intraoral ultrasound for preoperative assessment of depth of invasion for early tongue squamous cell carcinoma: Radiological-pathological correlations. Int J Environ Res Public Health. 2022 Nov 12;19(22):14900. https://doi.org/10.3390/ijerph192214900
-
Cherniak OS, Fesenko II. A rare odontogenic subperiosteal abscess that involved entire lateral aspect of the mandibular body and medial aspect to the level of mylohyoid ridge: ultrasound examination. J Diagn Treat Oral Maxillofac Pathol. 2023 Feb 28;6(2):10-20. https://doi.org/10.23999/j.dtomp.2023.2.1
-
Demidov VH, Ripolovska OV. How multiple the submandubular gland sialoliths can be? J Diagn Treat Oral Maxillofac Pathol. 2019 Jul;3(7):174-175. https://doi.org/10.23999/j.dtomp.2019.7.2
-
Dawkins A, Sobieh A, Nair R, Ganesh H. Contemporary sonography of the floor of the mouth: Anatomic highlights, pitfalls, and normal variants. Contemp Diagn Radiol. 2022 Mar 15;45(6):1-5. https://doi.org/10.1097/01.CDR.0000824016.86045.a5
-
Rozylo-Kalinowska I, K. Orhan K. General considerations for ultrasound applications in head and neck. In: Ultrasonography in dentomaxillofacial diagnostics. Orhan K, editor. Springer Nature Switzerland AG; 2021:39-50. https://doi.org/10.1007/978-3-030-62179-7_3
-
Kim K, Oh D, Shin D, Yoon J. Effectiveness of a hockey-stick probe to localize a catheter fragment in a dog. J Vet Clin. 2022 Aug 31;39(4):173-176. https://doi.org/10.17555/jvc.2022.39.4.173
-
Fu L, Chang JJ, Al Hezaimi K, et al. In vivo periodontal ultrasound imaging via a hockey-stick transducer and comparison to periodontal probing: a proof-of-concept study. Clin Oral Investig. 2025 Apr 26;29(5):275. https://doi.org/10.1007/s00784-025-06346-w
-
Caglayan F, Bayrakdar IS. The intraoral ultrasonography in dentistry. Niger J Clin Pract. 2018 Feb;21(2):125-33.http://dx.doi.org/10.4103/1119-3077.197016
-
Cheng ET, Greene D, Koch RJ. Metastatic renal cell carcinoma to the nose. Otolaryngol Head Neck Surg. 2000;122(3):464. https://doi.org/10.1016/s0194-5998(00)70069-6
-
Hirshberg A, Berger R, Allon I, Kaplan I. Metastatic tumors to the jaws and mouth. Head Neck Pathol. 2014;8(4):463-474. https://doi.org/10.1007/s12105-014-0591-z
-
Martins FMC, Neto TH, Martins SP, Mendes RB. Metastasis to the oral cavity and jaw bones - A literature review about a case. Oral Oncol. 2023;137:106276. https://doi.org/10.1016/j.oraloncology.2022.106276
-
Küsters B, Westphal JR, Smits D, et al. The pattern of metastasis of human melanoma to the central nervous system is not influenced by integrin alpha(v)beta(3) expression. Int J Cancer. 2001 Apr 15;92(2):176-180. https://doi.org/10.1002/1097-0215(200102)9999:9999%3C::aid-ijc1173%3E3.0.co;2-l
ARTICLE TITLE IN UKRAINIAN
Звіт про випадок метастазу світлоклітинної карциноми нирки в ротовій порожнині, що імітував доброякісне новоутворення: можливості та обмеження ультразвукового дослідження
SUMMARY IN UKRAINIAN
Метастази в ротовій порожнині трапляються рідко, складаючи приблизно 1-1,5% усіх злоякісних новоутворень ротової порожнини. Тому клініцистам важливо напрацьовувати діагностичні дані для ранньої діагностики цього грізного патологічного процесу. Найпоширенішими первинними пухлинами, що метастазують у ротову порожнину, є: легені (21,1%), печінка (12,3%), молочна залоза (10,5%), нирки (10,5%) та колоректальна пухлина (8,8%). Ми сподіваємося, що публікація описаного нижче випадку доповнить міжнародну літературу та виведе ультразвукову діагностику на новий рівень. На хірургічне обстеження звернулася 49-річна жінка європеоїдної раси зі зростаючим новоутворенням у ротовій порожнині та його грибоподібним прикріпленням до слизової оболонки альвеолярного відростка нижньої щелепи. Новоутворення клінічно імітувало доброякісну патологію. Новоутворення (його передня частина) було досліджено за допомогою ультразвукового дослідження (УЗД) з представленням сірошкальних та колірових Допплерівських сонограм. Варто відзначити що вестибулярна (передня, менша) частина метастазу в даному випадку не мала характерної ознаки для метастазів в інших ділянках тіла, а саме була відсутня васкуляризація при колірному Допплерівському картуванні. У статті також наведено клінічні дані та дані ортопантомографії. Було проаналізовано обмеження лінійного датчика для повної ультразвукової оцінки новоутворень, розташованих на язичному або, можливо, піднебінному боці альвеолярних відростків. Представлено порівняльний аналіз лінійного ультразвукових датчика та лінійного датчика типу «хокейна ключка». Після проведення аналізу літератури можна стверджувати, що в цій статті вперше представлені дані щодо УЗД метастазів світлоклітинної карциноми нирки на слизовій оболонці рота. Подальше дослідження кожного метастазу в ротовій порожнині за допомогою прямого внутрішньоротового УЗД є надзвичайно важливим для систематизації ультразвукових варіантів цієї патології. У свою чергу, це дозволить клініцистам з більшою ймовірністю встановити правильний попередній діагноз перед біопсією та лікувати цю патологію у спеціалізованих відділеннях.
KEY WORDS IN UKRAINIAN
Метастаз в ротову порожнину, світлоклітинна карцинома нирки, ультразвукове дослідження (УЗД), лінійний датчик, лінійний датчик «хокейна ключка»