November 30, 2022
J Diagn Treat Oral Maxillofac Pathol 2022;6: 138–47.
Under a Creative Commons license
HOW TO CITE THIS ARTICLE
Demidov VH, Cherniak OS, Snisarevskyi PP, Zaritska VI, Shatrova KM. Schwannoma of the tongue: ultrasonography. J Diagn Treat Oral Maxillofac Pathol 2022;6(11):138–47. https://doi.org/10.23999/j.dtomp.2022.11.2
NATIONAL REPOSITORY OF ACADEMIC TEXTS
https://nrat.ukrintei.ua/en/searchdoc/2022U000199/
SUMMARY
Schwannoma is a rare benign tumor originated from the Schwann cells of the nerve sheath. Other common names are neurilemmoma, neurinoma, and neurinoma of Verocay. The tumor is encapsulated and shows slow growth reaching even the 8.5-cm size. The purpose of this paper is to provide clinical presentation, sonogram and ultrasound video of schwannoma of the tongue, its analysis along with intraoperative and histopathological data. A 27-year-old female patient with tongue schwannoma is presented and analysis of the published schwannoma cases in different anatomical areas is performed. Distinctive sonographic features of this type of tumor are showed and comparison with the other tongue masses is highlighted. Ultrasonography proved its efficacy as a first-line diagnostic tool which needs to be popularized among oral and maxillofacial surgeons.
INTRODUCTION
Schwannoma is a rare benign tumor originated from the Schwann cells of the nerve sheath.1,2 Other common names are neurinoma2, neurinoma of Verocay,3 and neurilemmoma3. The tumor is encapsulated and shows slow growth reaching even 8- or 8.5-cm size.2,4
In contrast to other studies, which demonstrate data from magnetic resonance imaging (MRI) examination1,5,6 in schwannomas and a limited number of ultrasound (US) images6, the purpose of this article is to provide video imaging of schwannoma of the tongue, its analysis along with clinical, intraoperative, and histopathological data.
CASE
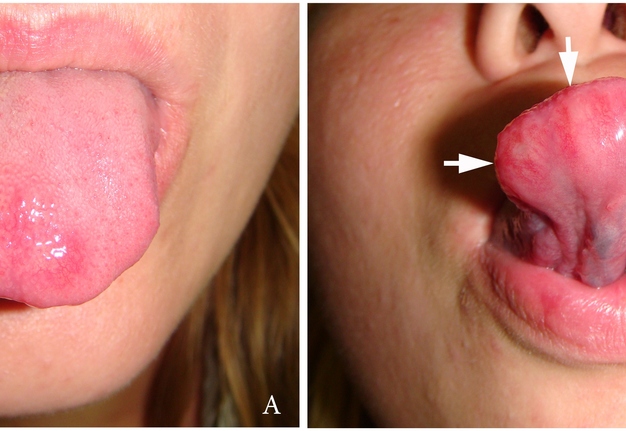
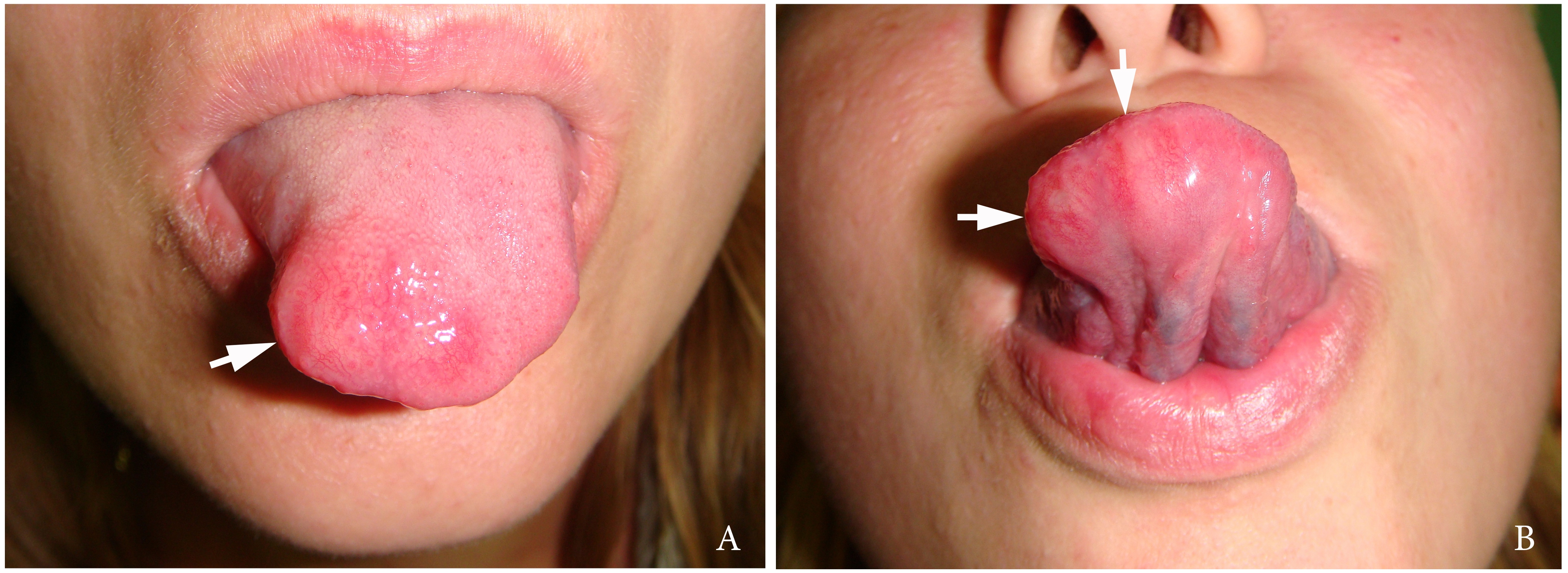
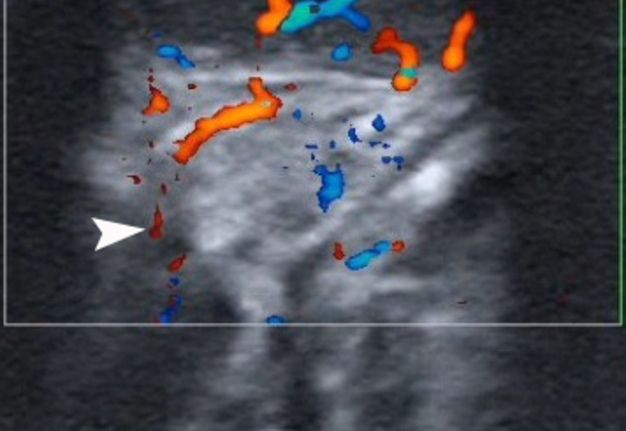
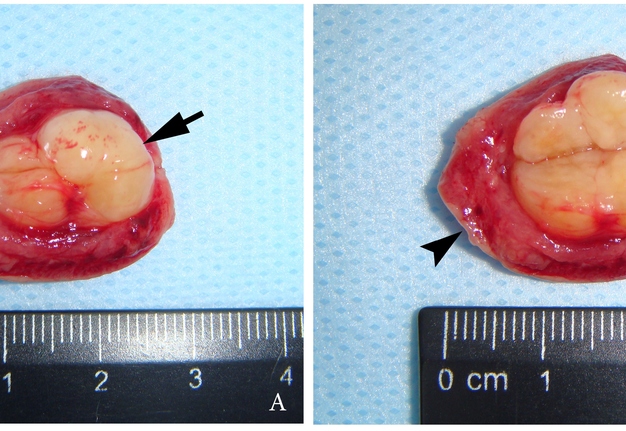
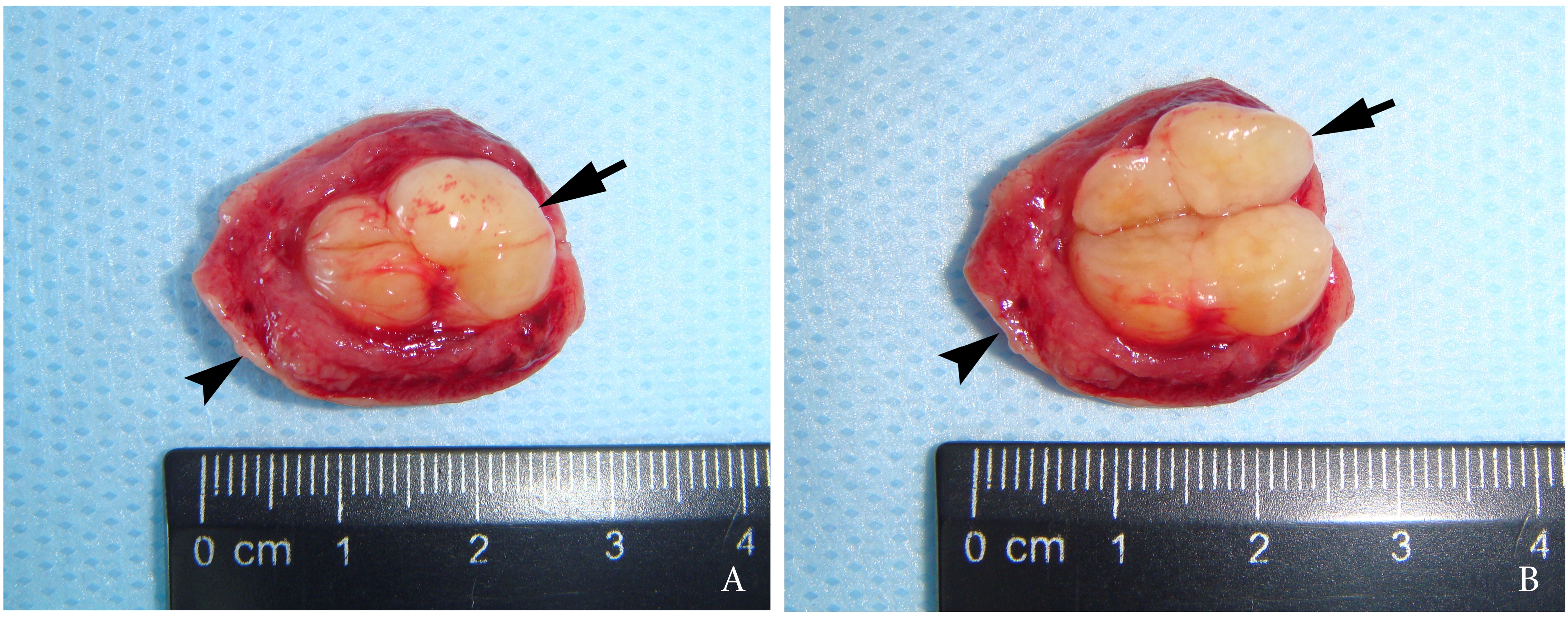
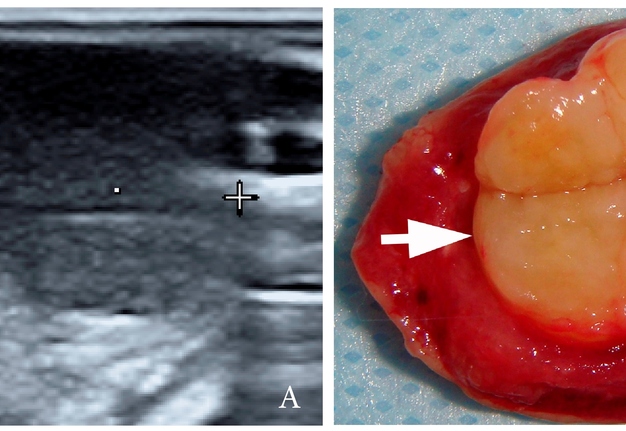
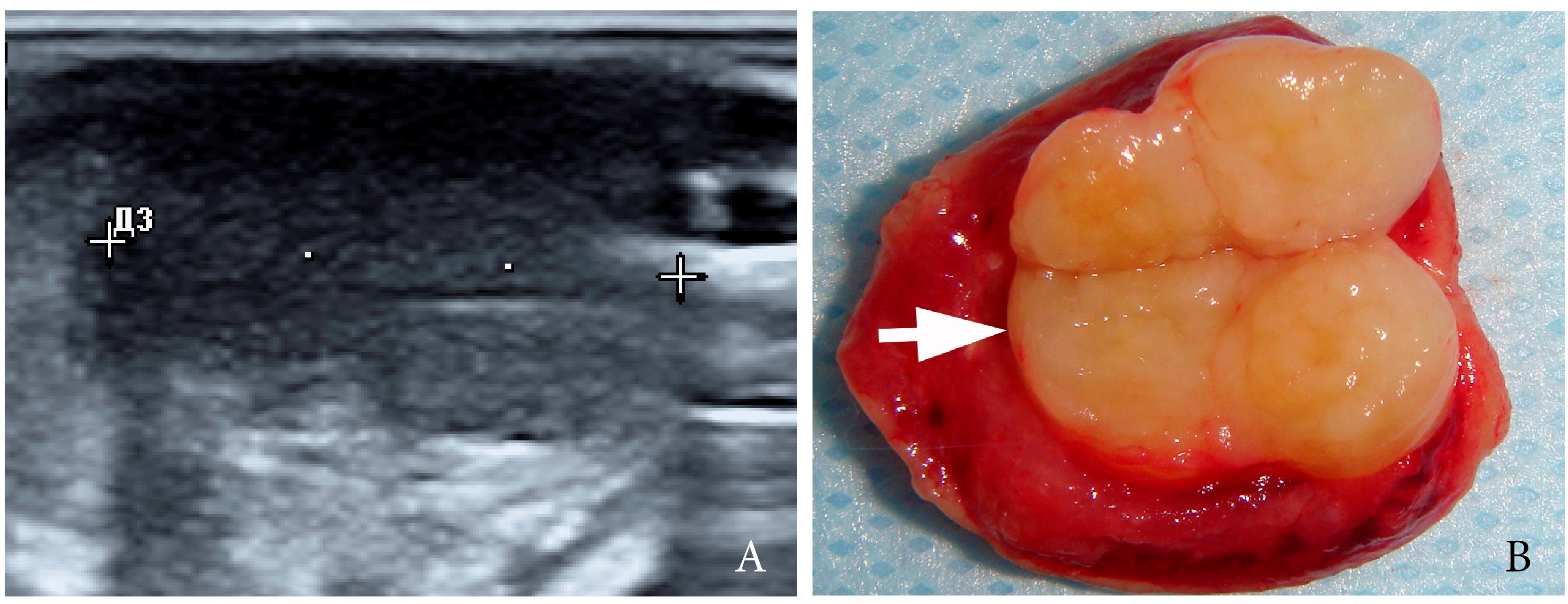
A 27-year-old female patient presented to the Center of Maxillofacial Surgery and Dentistry in 2016 with tongue asymmetry due to the expansive lesion (Fig 1). The lesion had been growing insidiously for last several years. Tongue examination revealed a firm, soft, lobulated mass on the right tongue, painless upon palpation. Overlying mucosa was slightly erythematous. Real-time gray scale and color Doppler US with its interpretation has been done by O.S.C. (her experience in head and neck US is 16 years). US was performed with the patient in the supine position, sticking out the tongue, and using an Esaote MyLabTM Seven machine (Esaote S.p.A, Genoa, Italy) and a 3- to 19-MHz linear array transducer, wrapped in a plastic film. The patient was informed that the gel (ECO SUPERGEL, Ceracarta S.p.A., Forlì, Italy) used for US diagnostics is nontoxic. Gray-scale US (Fig 2) of the right tongue showed bilobed well-defined hypoechoic and slightly heterogeneous lesion (with dense echoic graininess). The lesion was hypoechoic relative to adjacent tongue muscles and weakly compressible. Long-to-short diameter of the lesion measured 1.69 × 0.9 cm. The artifact of acoustic enhancement behind the lesion, edge artifact, and reverberation lines (i.e., the reverberation artifact) are visualized on the sonogram. No anechoic areas typical for cystic areas are visualized. Absence of the parallel echoic streaks typical for the majority of lipomas, presence of acoustic enhancement, and weak compressibility made this US appearance uncommon for the lipomas.7,8 Video (Supplemental Video Content) demonstrates color Doppler US of the right side of the tongue’s body. Tumor visualized as a heterogeneously hypoechoic mass. The prominent intratumoral vascularization and vascular motion artifact was noted. Video is available in the page of the full-text article on www.dtjournal.org and in the YouTube channel, available at https://youtube.com/shorts/qFO18Mnd-pQ?feature=share. Total video’s duration is 22 seconds. Surgical excision included a margin of adjacent tissue and was performed under the general anesthesia. Specimen (Fig 3) visualized as a resected part of the tonguewith a bilobed (dumbbell-shaped) yellow-white tumor inside. The tumor had well-circumscribed margins and was slightly tender on palpation. Macroscopically, the color of tumor mimicked lipoma. Figure 4 compares the tumor’s appearance on gray scale sonogram and macroscopically. The patient experienced no neurological disorder postoperatively.
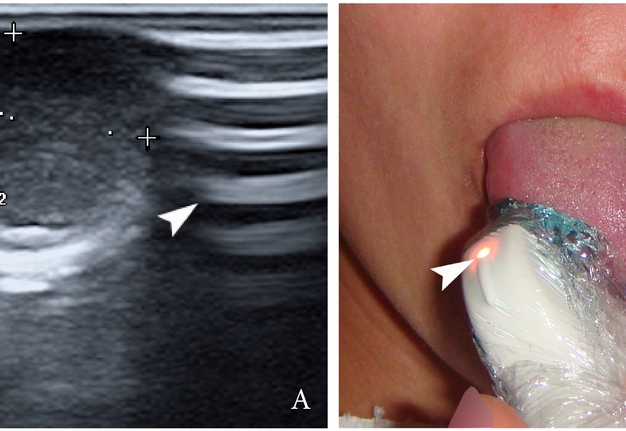
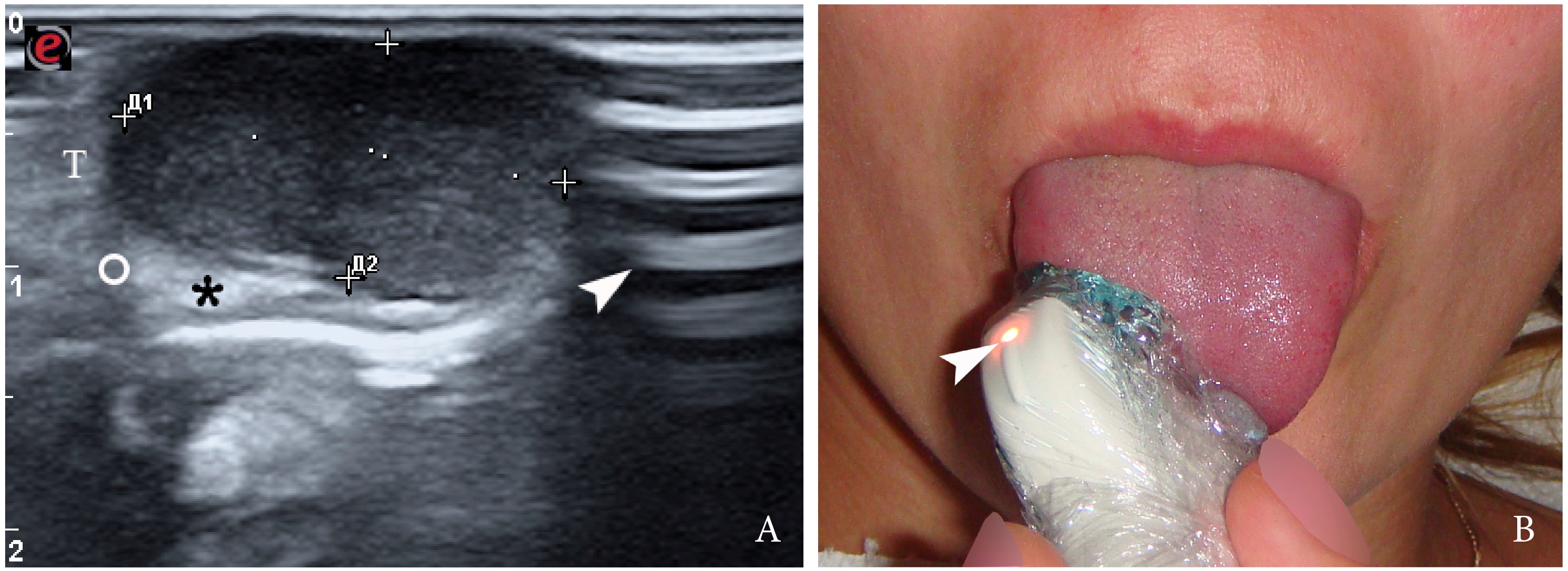
FIGURE 2. Gray-scale ultrasound (A) of the right tongue shows lobulated well-defined hypoechoic and slightly heterogeneous lesion (with dense echoic graininess). The lesion is hypoechoic relative to adjacent tongue (T) muscles and is weakly compressible. Long-to-short diameter of the lesion measured 1.69 × 0.9 cm (long diameter is indicated by Cyrillic letter with number (Д1) and two ‘+’ calipers in a horizontal direction; short diameter is indicated by Cyrillic letter with number (Д2) and two ‘+’ calipers in a vertical direction). The artifact of acoustic enhancement (asterisk) behind the lesion, edge artifact (circle), and reverberation lines (i.e., the reverberation artifact) (arrowhead) are visualized on the sonogram. No anechoic areas typical for cystic areas are visualized. Absence of the parallel echoic streaks typical for the majority of lipomas, presence of acoustic enhancement, and weak compressibility make this US appearance uncommon for the lipomas.7,8 The “depth” of cropped gray-scale sonogram is 2.0 cm. Red letter “e” at the upper left corner of the sonogram indicates on the probe’s side (corresponds to the probe bump and light [arrowhead]). B, position of the probe upon ultrasonography. Printed with permission and copyrights retained by O.S.C.
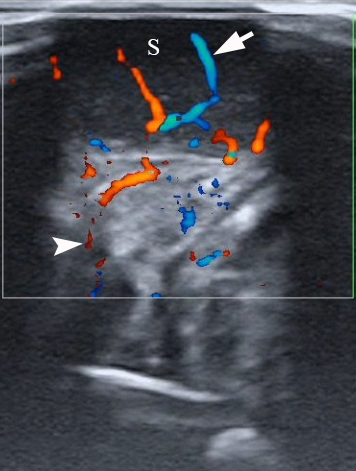
VIDEO. A 27-year-old female patient. Supplemental Video Content demonstrates color Doppler ultrasound of the schwannoma (S) of the tongue. The tumor is visualized as a heterogeneously hypoechoic mass. The intratumoral vascularization (arrow) and vascular motion artifact (arrowhead) are noted. The “depth” of cropped US image is 3.0 cm. Video is available in the page of the full-text article on www.dtjournal.org and in the YouTube channel, available at https://youtube.com/shorts/qFO18Mnd-pQ?feature=share.
Total video’s duration: 22 seconds. This video is a repeated ten-second recording of color Doppler ultrasound that is repeated for better visualization.
Printed with permission and copyrights retained by O.S.C.
FIGURE 3. Macroscopic view of the specimen before (A) and after (B) the incision of the bilobed intramuscular tumor. Notes the resected part of the tongue (arrowhead) and yellow-white tumor (arrow) inside. The tumor had well-circumscribed margins and was slightly tender on palpation. Macroscopically, the color of this schwannoma mimicking lipoma.
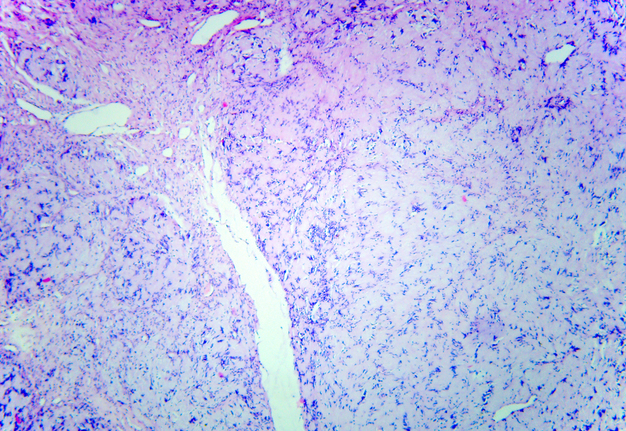
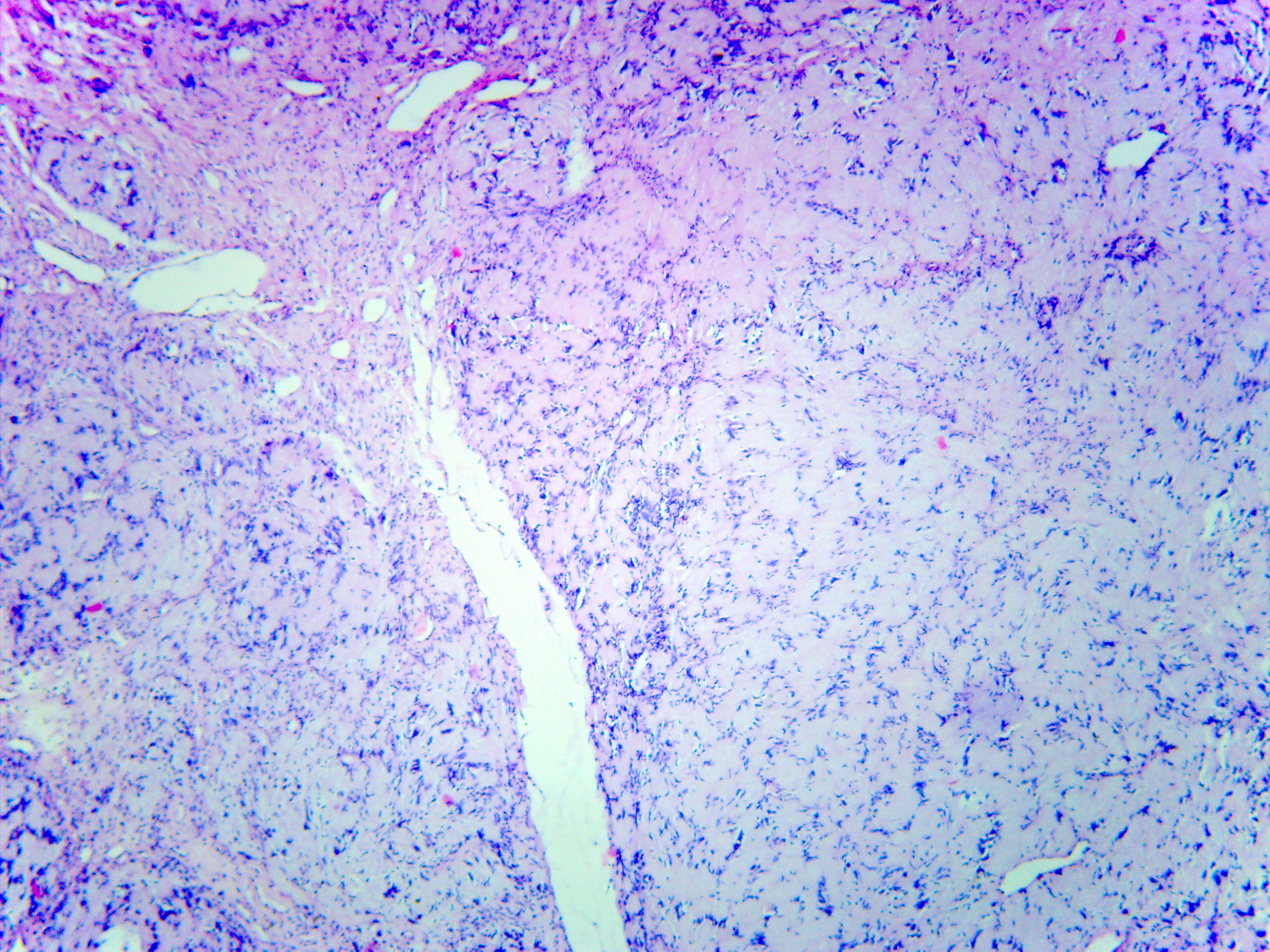
Schwannoma was established as histopathological diagnosis (Fig 5) by three experienced doctor-pathologists (P.P.S., his experience is 18 years; V.I.Z., her experience is 23 years; K.M.S., her experience is 48 years). No recurrence has been observed in a 2-year follow-up period.
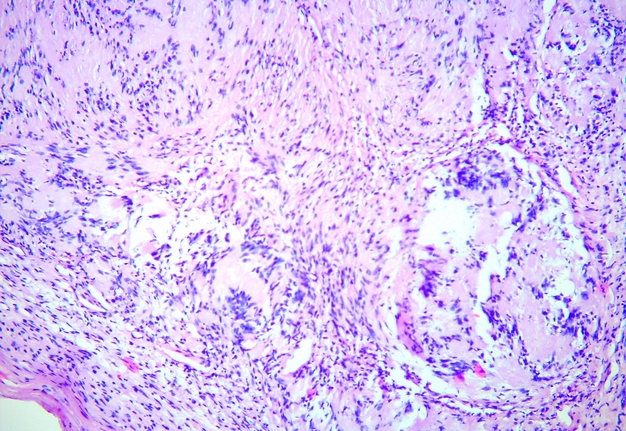
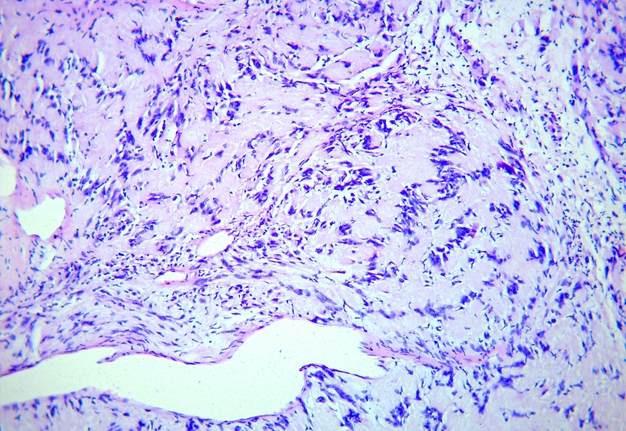
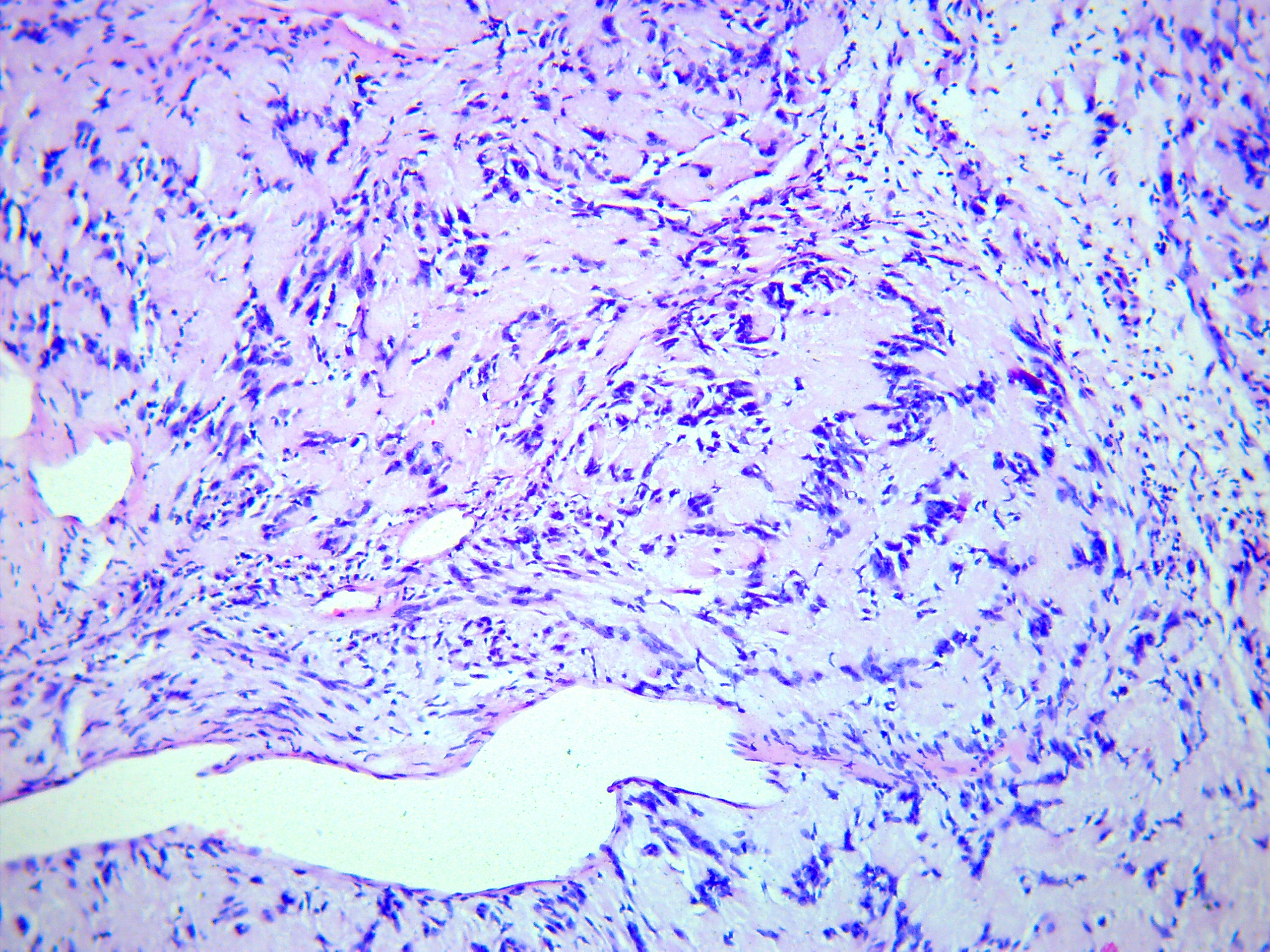
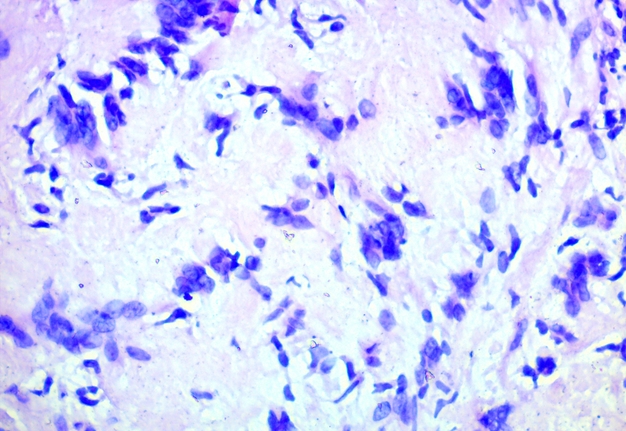
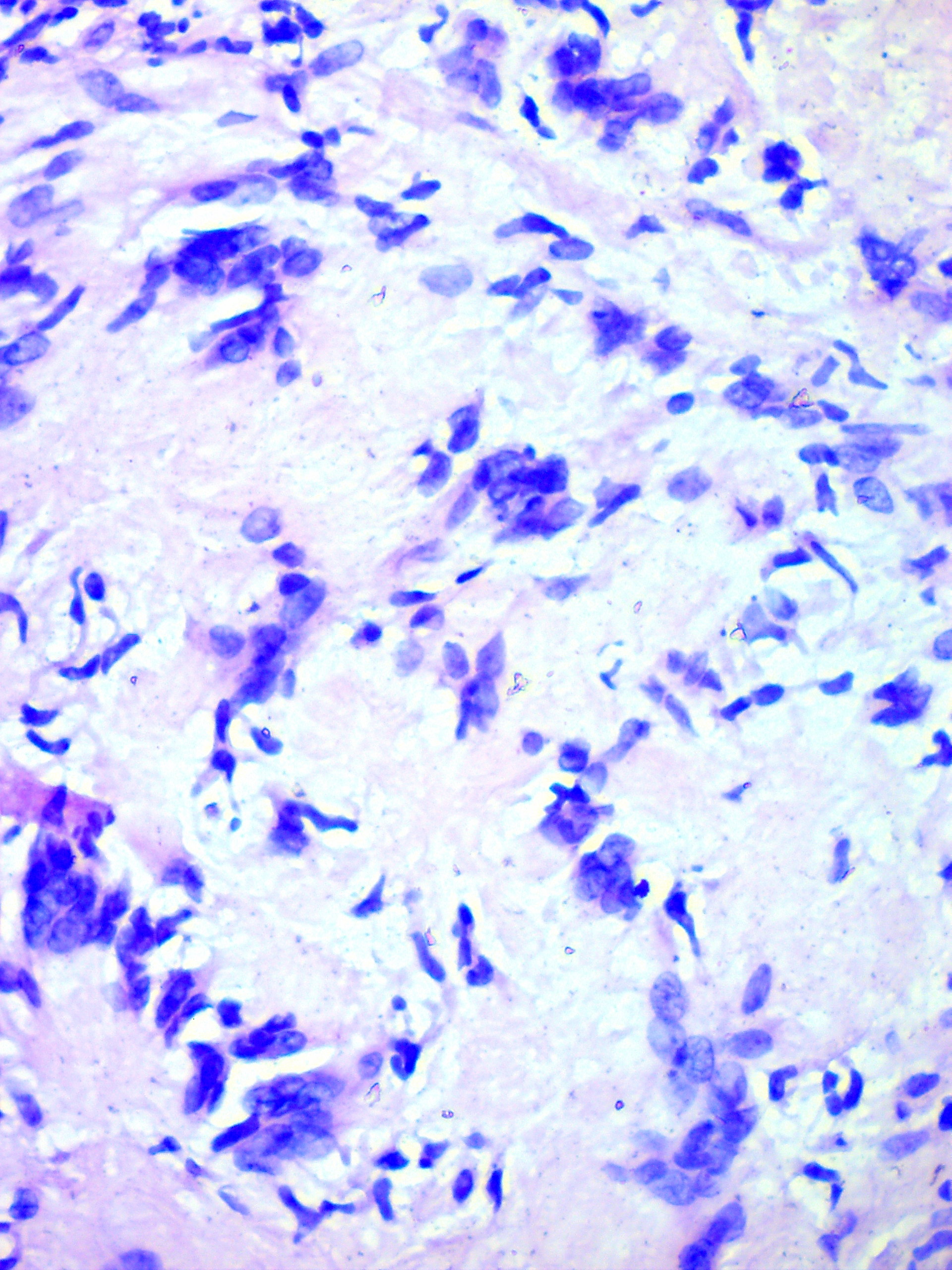
FIGURE 5A. Microscopic view of the presented schwannoma of the tongue. Fibrous structures are grouped into bundles that are placed randomly or in some places form formations with “swirls” and numerous correctly oriented cells that form palisade and pseudopalisade-like structures (Verocay bodies). Hematoxylin and eosin stain. Magnification: A, ×40; B–D, ×100; E, ×400.
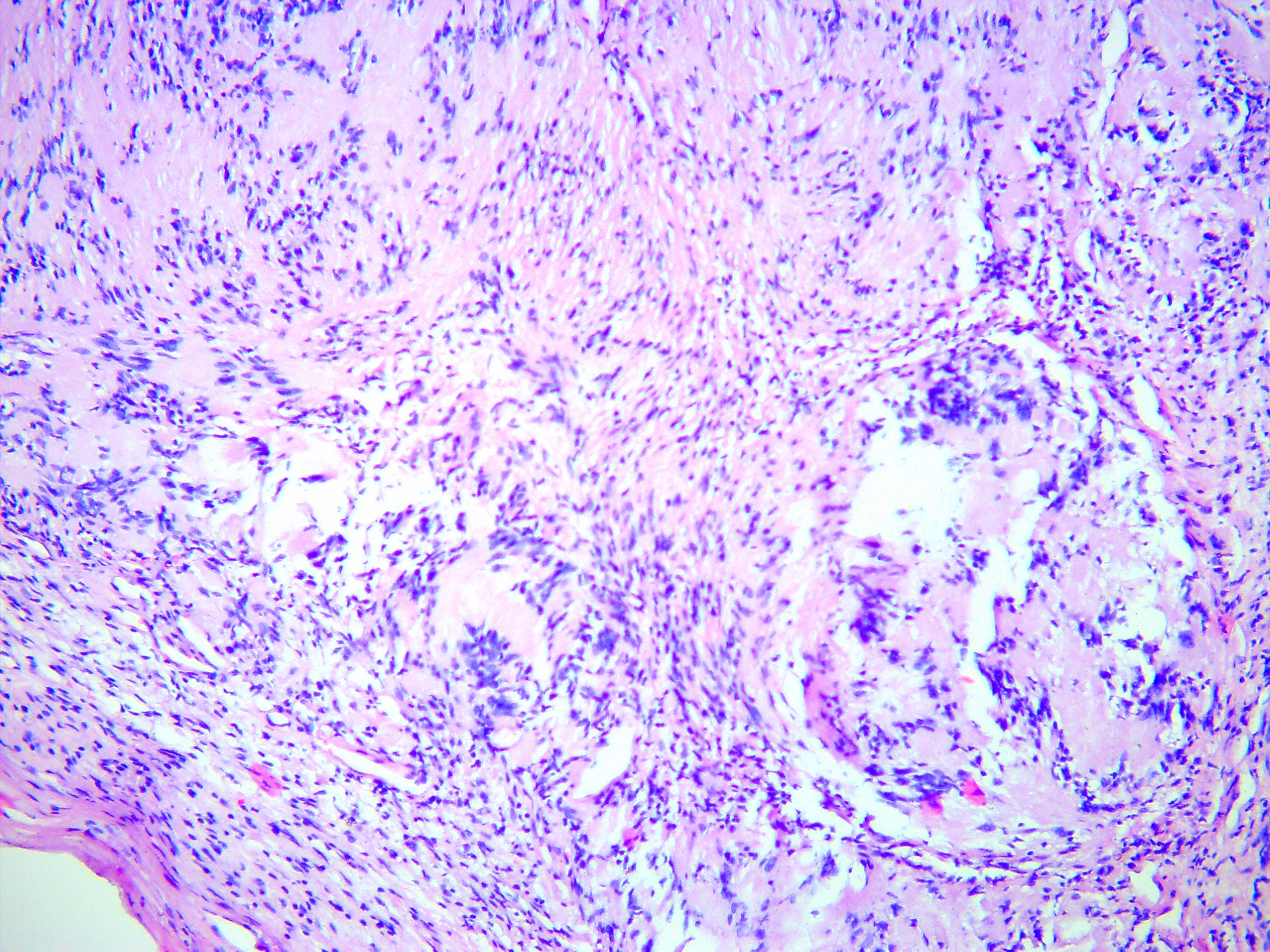
FIGURE 5B. Microscopic view of the presented schwannoma of the tongue. Fibrous structures are grouped into bundles that are placed randomly or in some places form formations with “swirls” and numerous correctly oriented cells that form palisade and pseudopalisade-like structures (Verocay bodies). Hematoxylin and eosin stain. Magnification: A, ×40; B–D, ×100; E, ×400.
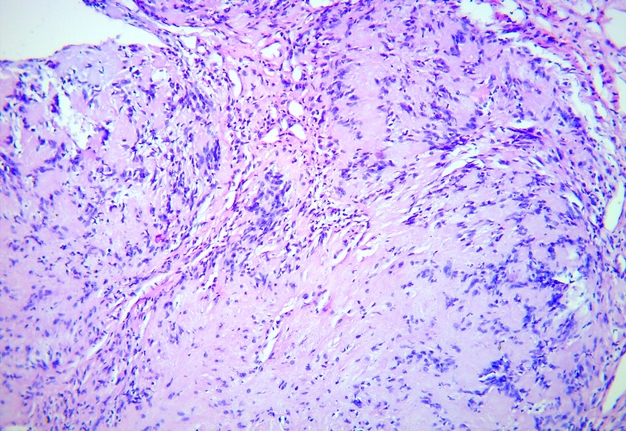
FIGURE 5C. Microscopic view of the presented schwannoma of the tongue. Fibrous structures are grouped into bundles that are placed randomly or in some places form formations with “swirls” and numerous correctly oriented cells that form palisade and pseudopalisade-like structures (Verocay bodies). Hematoxylin and eosin stain. Magnification: A, ×40; B–D, ×100; E, ×400.
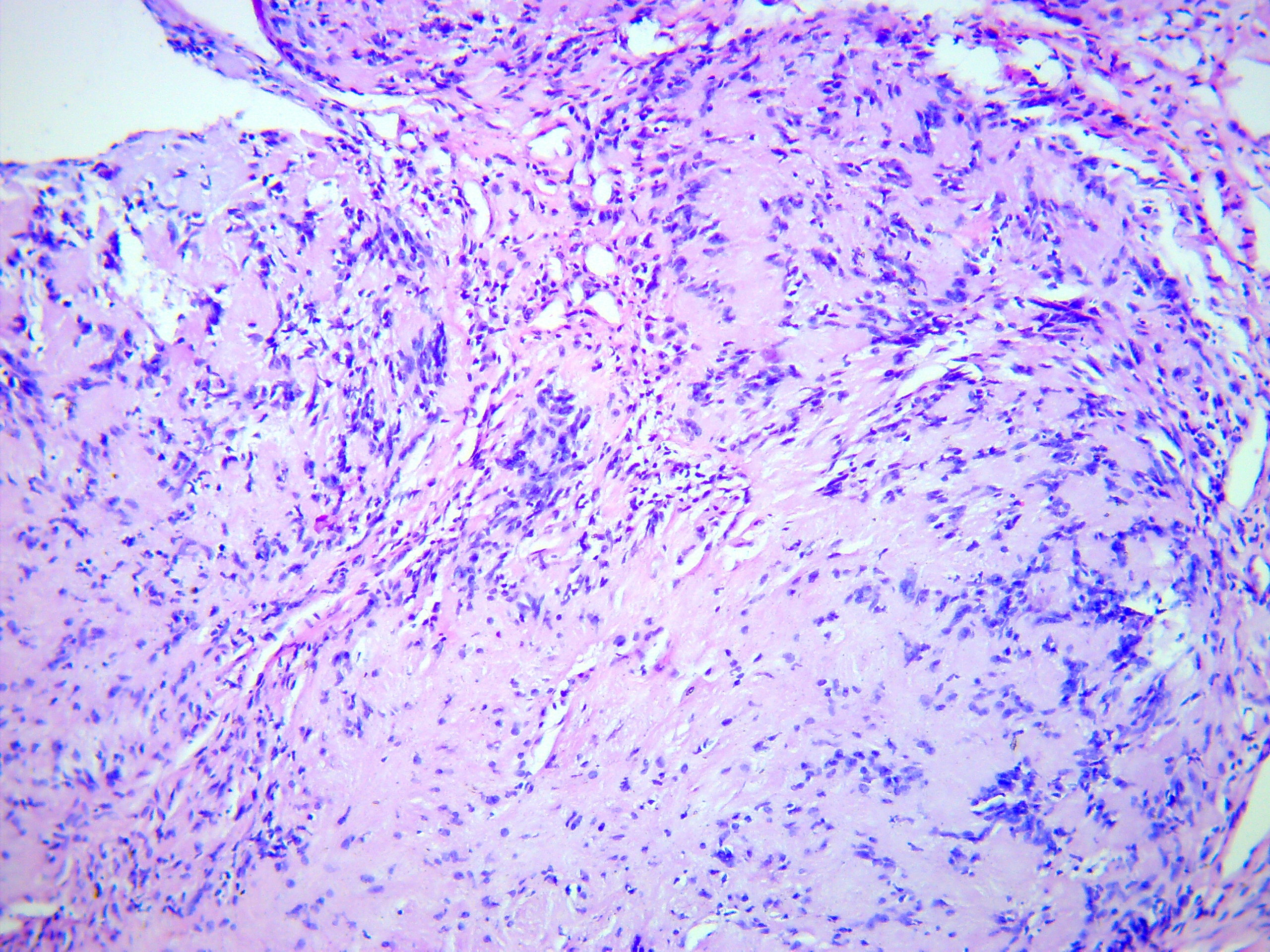
FIGURE 5D. Microscopic view of the presented schwannoma of the tongue. Fibrous structures are grouped into bundles that are placed randomly or in some places form formations with “swirls” and numerous correctly oriented cells that form palisade and pseudopalisade-like structures (Verocay bodies). Hematoxylin and eosin stain. Magnification: A, ×40; B–D, ×100; E, ×400.
FIGURE 5E. Microscopic view of the presented schwannoma of the tongue. Fibrous structures are grouped into bundles that are placed randomly or in some places form formations with “swirls” and numerous correctly oriented cells that form palisade and pseudopalisade-like structures (Verocay bodies). Hematoxylin and eosin stain. Magnification: A, ×40; B–D, ×100; E, ×400.
DISCUSSION
According to Kang et al (2007), neurilemmomas (i.e., schwannomas) may originate from any peripheral, cranial or autonomic nerves of the body (excepte of the olfactory and the optic nerves).9 Schwannomas reported in pancreas10, kidneys11, hands12, thumbs13, floor of the mouth14,15, soft palate16, maxillary alveolus17, tongue4,18-19, etc. Pfeifle et al (2021) indicate that total of 25 percent of neurilemmomas occur in the head and neck area, but only 1 percent are intraorally located.20
Analysis performed by Kavčič and Božič (2016) revealed that schwannomas in tongue are noted in one of two locations―base (one-third of cases) and oral part (two-thirds of cases).21
The analysis of the sonographic appearances of other tongue masses is well described in the literature.6 Konishi et al (2020) showed how huge the potential of tongue ultrasonography is, presenting the US examination of patients with primary tongue cancer.22 Sugawara et al (2016) depicted the US appearance of different pathologies involving tongue―fibrous polyp, cavernous haemangioma, pyogenic granuloma, lipoma, liposarcoma, chondroma, lymphangioma, schwannoma, solitary neurofibroma, pleomorphic adenoma, and amyloidosis.6
The case of schwannoma presented by Sugawara et al (2016) highlighted MRI, B-mode and power Doppler US, and histopathological features of the tumor in a 16-year-old male.6 Gray-scale US in their case showed an elliptical mass with a well-defined border and a comparatively homogeneous echo texture with posterior acoustic enhancement.6 Power Doppler showed motion artifacts and did not allow to evaluate the tumor vascularity.6 In our case, despite the motion artifacts, a prominent intratumoral vascularity was noted.
Kang et al (2007) performed retrospective analysis of twenty one head and neck schwannomas managed in a single institution. Their study showed one case of the US appearance of the schwannoma.9 It was an accessory nerve schwannoma with a supraclavicular location which was visualized on gray-scale US as non-vascular well-defined cystic lesion containing dependant debris.9
Cases presented in the reports of Pfeifle et al (2001)20 and Kavčič and Božič (2016)22, comparing with our case, demonstrate similar clinical presentation of the tongue schwannoma.
Describing the US features of the schwannoma, in our case we should apply the US features/criteria of the Ahuja et al (1998) for the differential diagnosis with head and neck lipomas.7 (1) Echogenicity, (2) shape and size, (3) internal architecture, (4) border, (5) distal enhancement, (6) compressibility, and (7) color flow are those features/criteria.7
Elliptical (88 percent) and ovoid (12 percent) shape is typical for lipomas7, but in our case the shape was lobulated. The fundamental sonographic study of Zhong et al (2004) showed that in 90 percent of cases the lipomas are hypoechoic with either echogenic spots or lines, and in 10 percent lipomas are either isoechoic or hyperechoic.8 So, according to Zhong et al (2004) and Ahuja et al (1998) sonographic appearance of lipomas is with echogenic spots/lines noted from 90 to 100 percent of cases.8,7 In our report, the tumor was hypoechoic, what corresponds to only 16 percent7 of lipomas. Clearly sonographically identified capsule of lipomas is noted in 88 percent of cases.7 Typically, none of the investigated lipomas showed an artifact of posterior enhancement or attenuation7, but the presented tumor showed prominent acoustic enhancement. All lipomas are compressible with moderate probe pressure.7 Usually, head and neck lipomas showed no internal vascularity7, and in our case the prominent intratumoral vascularity and a vascular motion artifact was noted. Recognition of a vascular motion artifact and artifact of acoustic enhancement is important, as its understanding will help in US examination of the tissues and tumor’s structure thus facilitating the differential diagnostics (Hindi et al, 2013).23
In summary, among all seven US features/criteria (Ahuja et al, 1998), the tumor in our case was positive only in one feature (hypoechoic echogenicity).7
The profound review of the publications dedicated to tongue neurilemmomas published from 1955 to 2016 was performed by Lee et al (2017).12
In our opinion, it is not correct to draw conclusions based on US patterns of schwannoma only in the area of the tongue, but it is worth knowing the options of US pictures of schwannoma in other anatomical areas. This will make possible understanding the entire palette of possible US patterns for schwannoma, which in turn will facilitate differential diagnosis and increase the accuracy of establishing a preoperative diagnosis and choosing the most appropriate treatment tactics.
Summarizing sonographic appearances of the schwannoma in different anatomical locations worth to note that possible US patterns are solid6,24,25, cystic9,12,26, and mixed27,28. Reynolds et al (2003) presented the US results more than worth of attention.24 Their team made sonographic comparison of two types of peripheral nerve sheath tumors—schwannomas and neurofibromas.24 In 83 percent the schwannomas were hypoechoic, 67 percent of schwannomas were homogeneous, 67 percent of neurilemmomas showed artifact of posterior acoustic enhancement, and 67 percent of neurilemmomas were centrally related to the involved peripheral nerve.24
Increased flow on color Doppler US can be noted in the schwannoma cases (Beggs,29 1999; Reynolds et al,24 2003). These data are supported by our case.
Unlike schwannomas of the tongue, schwannomas of the neck are characterized by thickening of the adjacent nerve.30 In 2012, Ahuja and Yuen indicated that despite some differences, US cannot reliably distinguish schwannomas of the neck from neurofibromas.28 However, even mild probe’s pressure obliterates schwannoma’s vascularity, what indicates that neurilemmomas vascularity is very sensitive to pressure.30
Shrikrishna et al (2016) published case series which included vagal schwannoma, axillary nerve schwannoma, and cervical sympathetic chain schwannoma.31 In their article, the preoperative view, computed tomography, intraoperative view, and histopathology are presented, allowing to compare schwannomas derived from different nerves in different locations.31
Harazano et al (2022) presented retrospective histopathological analysis of forty schwannomaslocalized in the oral and maxillofacial region.32 Among the anatomical areas of the schwannoma cases presented in their study were—tongue (45 percent), lower lip (17.5 percent), hard palate (10 percent), parotid gland (5 percent), buccal mucosa (7.5 percent), mandible (7.5 percent)33, floor of the mouth (2.5 percent), maxilla (2.5 percent), and cheek (2.5 percent).32
The existed and described histopathological variants are: two main patterns (Antoni A and B) (i.e., classic schwannoma) and multiple subtypes—ancient, plexiform, cellular, andepithelioid.32 Ancient (i.e., degenerative) neurilemmomas are uncommon in oral and maxillofacial area.32 It’s important to know that schwannomas can have pathological characteristics of both main patterns and ancient changes or can show no evidence of Antoni A and B patterns and degenerative subtype.32 In general, the authors categorized histopathological combinations into 8 variants.32
Our case supports the thesis of Winter et al (2020) who emphasized that US is a well-established, non-invasive, and easily repeatable first-line tool in diagnostics of soft tissue tumors.34
CONCLUSIONS
Our report demonstrated unique clinical images, sonograms and ultrasound video of schwannoma of the tongue which is macroscopically presented as a bilobed solid well-circumscribed tumor. More ultrasound descriptions of tongue schwannoma cases are needed to create a wider picture of possible echo-patterns of this type of tumors in a tongue organ.
TERM OF CONSENT
Writing patient’s consent was obtained for publication the photos.
AUTHOR CONTRIBUTIONS
Conceptualization: Demidov VH, Cherniak OS. Ultrasonographic data acquisition: Cherniak OS. Surgical images acquisition: Demidov VH. Histological data acquisition: Zaritska VI, Snisarevskyi PP. Data analysis or interpretation: Cherniak OS, Zaritska VI, Shatrova KM. Drafting of the manuscript: Cherniak OS. Critical revision of the manuscript: Cherniak OS, Demidov VH, Zaritska VI,Snisarevskyi PP. Approval of the final version of the manuscript: all authors.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
FUNDINGS
No funding was received for this study.
REFERENCES (34)
-
Badar Z, Farooq Z, Zaccarini D, Ezhapilli SR. Tongue base schwannoma: differential diagnosis and imaging features with a case presentation. Radiol Case Rep 2016;11(4):336–40. Crossref
-
Tymofieiev OO. Manual of maxillofacial and dental surgery [in Russian]. 5th ed. Kyiv: Chervona Ruta-Turs; 2012:665–97.
-
Joshi R. Learning from eponyms: Jose Verocay and Verocay bodies, Antoni A and B areas, Nils Antoni andschwannomas. Indian Dermatol Online J 2012;3(3):215–9. Crossref
-
Lee EY, Kim JJ, Seok H, Lee JY. Schwannoma of the tongue: a case report with review of literature. Maxillofac Plast Reconstr Surg 2017;39(1):17. Crossref
-
Sousa AA, Porto JGW. Schwannoma of the oral tongue. Arch Head Neck Surg 2020;49:e00212020. Crossref
-
Sugawara C, Takahashi A, Kawano F, Kudo Y, Ishimaru N, Miyamoto Y. Intraoral ultrasonography of tongue mass lesions. Dentomaxillofac Radiol 2016;45(5):20150362. Crossref
-
Ahuja AT, King AD, Kew J, King W, Metreweli C. Head and neck lipomas: sonographic appearance. AJNR Am J Neuroradiol 1998;19(3):505–8.
-
Zhong LP, Zhao SF, Chen GF, Ping FY. Ultrasonographic appearance of lipoma in the oral and maxillofacial region. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98(6):738–40. Crossref
-
Kang GC, Soo KC, Lim DT. Extracranial non-vestibular head and neck schwannomas: a ten-year experience. Ann Acad Med Singap 2007;36(4):233–8.
-
Ntafam C, Miller AT, Beutler BD, Bamporiki J, Sahakian AB, Cheng PM. Pancreatic schwannoma: case report, clinico-pathologic correlation, and review of the literature. Radiol Case Rep 2022;17(10):3504–10. Crossef
-
Kokura K, Watanabe J, Takuma T, Uketa S, Uemura Y, Uegaki M. A case of renal schwannoma. Urol Case Rep2022;45:102232. Crossref
-
Lee SJ, Yoon ST. Ultrasonographic and clinical characteristics of schwannoma of the hand. Clin Orthop Surg2017;9(1):91–5. Crossref
-
Refalo A, Mallina R. Benign schwannoma of the thumb; a diagnostic challenge. Radiol Case Rep 2021;17(3):477–80. Crossref
-
Tsushima F, Sawai T, Kayamori K, Okada N, Omura K. Schwannoma in the floor of the mouth: a case report and clinicopathological studies of 10 cases in the oral region. J Oral Maxillofac Surg Med Pathol 2012;24(3):175–9. Crossref
-
Shim SK, Myoung H. Neurilemmoma in the floor of the mouth: a case report. J Korean Assoc Oral Maxillofac Surg2016;42(1):60–4. Crossref
-
Sata N, Tsunoda A, Ono N, Inoshita A, Ikeda K. A case of soft palate schwannoma that developed with obstructive sleep apnea syndrome (OSAS). Otolaryngol Case Rep 2018;9:34–6. Crossref
-
Kandasamy S, Nathan RS, John RR. Neurilemmoma of maxillary alveolus: a rare case report and review of literature. J Pharm Bioallied Sci 2017;9(Suppl 1):S285–8. Crossref
-
Moreno-García C, Pons-García MA, González-García R, Monje-Gil F. Schwannoma of tongue. J Maxillofac Oral Surg 2014;13(2):217–21. Crossref
-
López-Jornet P, Bermejo-Fenoll A. Neurilemmoma of the tongue. Oral Oncol Extra 2005;41(7):154–7. Crossref
-
Pfeifle R, Baur DA, Paulino A, Helman J. Schwannoma of the tongue: report of 2 cases. J Oral Maxillofac Surg2001;59(7):802–4. Crossref
-
Kavčič J, Božič M. Schwannoma of the tongue. BMJ Case Rep 2016;2016:bcr2016215799. Crossref
-
Konishi M, Fujita M, Shimabukuro K, Wongratwanich P, Verdonschot RG, Kakimoto N. Intraoral ultrasonographic features of tongue cancer and the incidence of cervical lymph node metastasis. J Oral Maxillofac Surg2021;79(4):932–9. Crossref
-
Hindi A, Peterson C, Barr RG. Artifacts in diagnostic ultrasound. Rep Med Imaging 2013;6:29–48. Crossref
-
Reynolds DL Jr, Jacobson JA, Inampudi P, Jamadar DA, Ebrahim FS, Hayes CW. Sonographic characteristics of peripheral nerve sheath tumors. AJR Am J Roentgenol 2004;182(3):741–4. Crossref
-
Sohn YM, Kim SY, Kim EK. Sonographic appearance of a schwannoma mimicking an axillary lymphadenopathy. J Clin Ultrasound 2011;39(8):477–9. Crossref
-
Filipov I, Chirila L, Sandulescu M, Cristache CM. A predictable approach of a rare and frequently misdiagnosed entity: laryngeal nerve schwannoma. Healthcare 2022;10(1):59. Crossref
-
Tatar IG, Yilmaz KB, Arikok A, Bayar B, Akinci M, Balas S, Ergul Z, Ergun O, Hekimoglu B. Cystic schwannoma of the axillary region: imaging findings of a rare disease. Case report. Med Ultrason 2015;17(1):126–8. Crossref
-
Ahuja AT, Yuen HY. Benign clinical conditions in the adjacent neck. In: Ultrasound of the thyroid and parathyroid glands. Sofferman RA, Ahuja AT, editors. 1st edition. New York: Springer Science+Business Media; 2012:229–59. Crossref
-
Beggs I. Sonographic appearances of nerve tumors. J Clin Ultrasound 1999;27(7):363–8. Crossref
-
Ahuja AT. Lumps and bumps in the head and neck. In: Practical head and neck ultrasound. Ahuja AT, Evans R, editors. 1st edition. London: Greenwich Medical Media Limited; 2000:87–106.
-
Shrikrishna BH, Jyothi AC, Kulkarni NH, Mazhar MS. Extracranial head and neck schwannomas: our experience. Indian J Otolaryngol Head Neck Surg 2016;68(2):241–7. Crossref
-
Harazono Y, Kayamori K, Sakamoto J, Akaike Y, Kurasawa Y, Tsushima F, Sasaki Y, Harada H, Yoda T. Retrospective analysis of schwannoma in the oral and maxillofacial region: clinicopathological characteristics and specific pathology of ancient change. Br J Oral Maxillofac Surg 2022;60(3):326–31. Crossref
-
Delantoni A, Apostolos Sarafopoulos A. Sonographic anatomy and pathology extracranial nerves. In:Ultrasonography in dentomaxillofacial diagnostics. Orhan K, editor. Cham: Springer Nature Switzerland; 2021:89–97. Crossref
-
Winter N, Dohrn MF, Wittlinger J, Loizides A, Gruber H, Grimm A. Role of high-resolution ultrasound in detection and monitoring of peripheral nerve tumor burden in neurofibromatosis in children. Childs Nerv Syst 2020;36(10):2427–32. Crossref